Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation - PubMed (original) (raw)
Characterization of Ran-driven cargo transport and the RanGTPase system by kinetic measurements and computer simulation
Dirk Görlich et al. EMBO J. 2003.
Abstract
Here, we analyse the RanGTPase system and its coupling to receptor-mediated nuclear transport. Our simulations predict nuclear RanGTP levels in HeLa cells to be very sensitive towards the cellular energy charge and to exceed the cytoplasmic concentration approximately 1000-fold. The steepness of the RanGTP gradient appears limited by both the cytoplasmic RanGAP concentration and the imperfect retention of nuclear RanGTP by nuclear pore complexes (NPCs), but not by the nucleotide exchange activity of RCC1. Neither RanBP1 nor the NPC localization of RanGAP has a significant direct impact on the RanGTP gradient. NTF2-mediated import of Ran appears to be the bottleneck for maximal capacity of Ran-driven nuclear transport. We show that unidirectional nuclear transport can be faithfully simulated without the assumption of a vectorial NPC passage; transport receptors only need to reversibly cross NPCs and switch their affinity for cargo in response to the RanGTP gradient. A significant RanGTP gradient after nuclear envelope (NE) breakdown can apparently exist only in large cytoplasm. This indicates that RanGTP gradients can provide positional information for mitotic spindle and NE assembly in early embryonic cells, but hardly any in small somatic cells.
Figures
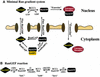
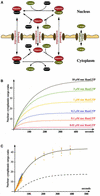
Fig. 1. Schematic overview of the RanGTPase system. (A) Ran switches between a GDP- and a GTP-bound form and circulates between nucleus and cytoplasm. Nucleotide exchange is catalysed by nuclear RCC1. Fluxes of RanGDP and RanGTP between nucleus and cytoplasm depend on their concentration differences and on the permeability of NPCs for RanGTP and RanGDP. The cytoplasmic conversion of RanGTP to RanGDP occurs either directly by RanGAP or after trapping of RanGTP by RanBP1. Receptor-mediated cargo transport occurs at the expense of the RanGTP gradient and results in a RanGTP transfer to the cytoplasm, where the bound nucleotide is hydrolysed to GDP. (B) Details of the nucleotide exchange reaction, which occurs in both directions and proceeds through three intermediates. E stands for the free RCC1 enzyme. r1-r8 are rate constants for the partial reactions (Klebe et al., 1995b).
Fig. 2. Description of the RanGTPase system by an ordinary differential equation system. Prefixes cyto and nuc stand for cytoplasmic and nuclear, respectively; fluxes of Ran between nuclear and cytoplasm are normalized to the nuclear volume. Other species and partial reactions are defined in Figure 1.
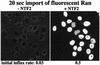
Fig. 3. Panels compare nuclear influx of RanGDP in the absence or presence of NTF2 (one homodimer per two RanGDP molecules). Quantitation of initial rates at 25°C revealed a permeability factor of NPCs towards free RanGDP of 0.03 s–1, i.e. for a nucleocytoplasmic concentration difference of 1 µM, the nuclear RanGDP concentration changes at 0.03 µM/s. The permeability factor for the NTF2–RanGDP complex is 0.48 s–1.
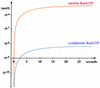
Fig. 4. Simulated time course for generation of the RanGTP gradient. At time zero of the simulation, all Ran was cytoplasmic and GDP bound. With these starting conditions, a nucleocytoplasmic RanGTP gradient builds up (half time ≈7 s). The figure shows a log linear plot for nuclear and cytoplasmic RanGTP concentrations. The following steady-state concentrations result: 4.3 µM nuclear RanGTP, 7.7 nM cytoplasmic RanGTP, 0.4 µM nuclear RanGDP and 1.5 µM cytoplasmic RanGDP. Parameters used for the simulation were: 1.2 pl nuclear volume, 1.8 pl cytoplasmic volume, 0.7 µM cytoplasmic RanGAP, 2 µM cytoplasmic RanBP1, 0.7 µM nuclear RCC1, 0.3 µM cellular NTF2 concentration (homodimers) and 3 µM cellular Ran concentration. For kinetic constants, see Supplementary tables and main text.
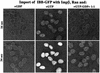
Fig. 5. Effect of GTP:GDP ratio on the rate of receptor-mediated nuclear accumulation. IBB–GFP (2 µM) fusion was complexed stoichiometrically with Impβ and imported into nuclei of permeabilized cells in the presence of a GTP-regenerating system, which keeps the GTP:GDP ratio >200, or with GDP, or with 500 µM GTP plus 500 µM GDP. The figure depicts, at indicated time points, the nuclear:cytoplasmic distribution of the import cargo, as visualized by confocal laser scanning microscopy.
Fig. 6. Dependence of substrate accumulation from the Ran gradient. (A) Scheme of the Impβ import system, representing the differential equation system used for import simulation. (B) Simulated import of an IBB–MBP fusion protein (1 µM) mediated by Impβ (0.5 µM). Parameters for the simulation were equivalent to experimental conditions of import into nuclei of permeabilized cells [i.e. the volume outside of the nuclei is very large compared with the nuclear volume; see also (C)]. Cytoplasmic RanGTP was fixed to 1 nM and nuclear RanGTP varied from 10 nM to 10 µM. For binding constants, see Supplementary table; see also Table IV. (C) Import of an IBB–MBP fusion into nuclei of permeabilized cells was performed at 37°C with the following components: 1 µM fluorescent IBB–MBP, 0.5 µM Impβ, 3 µM Ran, 0.4 µM NTF2 (dimers), 0.05 µM RanGAP, 0.1 µM RanBP1 and a GTP-regenerating system. The nuclear:cytoplasmic distribution of the import substrate is plotted for early time points in 2 s intervals and for later time points in 90 s intervals. Different colours indicate behaviour of individual nuclei in a typical experiment. Curves show simulated time course of nuclear IBB–MBP accumulation, whereby the mathematical model linked the generation of the RanGTP gradient (Figure 1) with transport at the expense of the gradient (Figure 6A). The simulation predicts 6 µM nuclear RanGTP at steady state. It reproduces time course and endpoint of nuclear cargo accumulation, provided the on-rate for RanGTP binding to Impβ is assumed not to be limiting (continuous curve). For that, RanGTP binding to Impβ must occur at least one to two orders of magnitude faster in the context of intact nuclei than observed with pure components. The dashed curve shows simulation using the on-rate of the RanGTP–Impβ interaction as measured with pure components (8·104 M–1s–1; Villa Braslavsky et al., 2000). The rates, by which the binding equilibria between cargo, RanGTP and Impβ are adjusted within the cytoplasm, are also not known. This uncertainty is, however, irrelevant for the cytoplasmic side of this simulation because the reaction volume outside nuclei is >1000-fold larger than the nuclear volume.
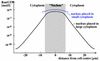
Fig. 7. Effect of the NE on the RanGTP gradient. Figure shows profile of simulated RanGTP levels within a HeLa cell. The nuclear area is represented in grey. The red curve shows interphase cell with intact NE and steep nucleocytoplasmic RanGTP gradient. The blue curve shows a mitotic cell lacking a NE; here, the RanGTP gradient has fully collapsed. For the mitotic situation, it was assumed that RCC1 was still chromatin bound, while RanGAP was evenly distributed between chromatin and cytoplasm.
Fig. 8. Effect of cytoplasm size on a mitotic RanGTP gradient. The RanGTP profile was simulated for a mitotic HeLa cell (blue curve) and for a cell with a larger cytoplasm (black curve). Note, only the larger cytoplasm allows the formation of a significant mitotic RanGTP gradient.
Similar articles
- Analysis of a RanGTP-regulated gradient in mitotic somatic cells.
Kaláb P, Pralle A, Isacoff EY, Heald R, Weis K. Kaláb P, et al. Nature. 2006 Mar 30;440(7084):697-701. doi: 10.1038/nature04589. Nature. 2006. PMID: 16572176 - Identification of different roles for RanGDP and RanGTP in nuclear protein import.
Görlich D, Panté N, Kutay U, Aebi U, Bischoff FR. Görlich D, et al. EMBO J. 1996 Oct 15;15(20):5584-94. EMBO J. 1996. PMID: 8896452 Free PMC article. - The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus.
Izaurralde E, Kutay U, von Kobbe C, Mattaj IW, Görlich D. Izaurralde E, et al. EMBO J. 1997 Nov 3;16(21):6535-47. doi: 10.1093/emboj/16.21.6535. EMBO J. 1997. PMID: 9351834 Free PMC article. - Structural biology of nucleocytoplasmic transport.
Cook A, Bono F, Jinek M, Conti E. Cook A, et al. Annu Rev Biochem. 2007;76:647-71. doi: 10.1146/annurev.biochem.76.052705.161529. Annu Rev Biochem. 2007. PMID: 17506639 Review. - The Ran GTPase as a marker of chromosome position in spindle formation and nuclear envelope assembly.
Hetzer M, Gruss OJ, Mattaj IW. Hetzer M, et al. Nat Cell Biol. 2002 Jul;4(7):E177-84. doi: 10.1038/ncb0702-e177. Nat Cell Biol. 2002. PMID: 12105431 Review.
Cited by
- Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity.
Schmidt HB, Görlich D. Schmidt HB, et al. Elife. 2015 Jan 6;4:e04251. doi: 10.7554/eLife.04251. Elife. 2015. PMID: 25562883 Free PMC article. - Nuclear roles for actin.
Wesolowska N, Lénárt P. Wesolowska N, et al. Chromosoma. 2015 Dec;124(4):481-9. doi: 10.1007/s00412-015-0519-8. Epub 2015 May 6. Chromosoma. 2015. PMID: 25944357 Review. - Crosstalk between the actin cytoskeleton and Ran-mediated nuclear transport.
Minakhina S, Myers R, Druzhinina M, Steward R. Minakhina S, et al. BMC Cell Biol. 2005 Aug 24;6:32. doi: 10.1186/1471-2121-6-32. BMC Cell Biol. 2005. PMID: 16120220 Free PMC article. - Use of virtual cell in studies of cellular dynamics.
Slepchenko BM, Loew LM. Slepchenko BM, et al. Int Rev Cell Mol Biol. 2010;283:1-56. doi: 10.1016/S1937-6448(10)83001-1. Int Rev Cell Mol Biol. 2010. PMID: 20801417 Free PMC article. Review. - On the nuclear pore complex and its emerging role in cellular mechanotransduction.
Matsuda A, Mofrad MRK. Matsuda A, et al. APL Bioeng. 2022 Mar 10;6(1):011504. doi: 10.1063/5.0080480. eCollection 2022 Mar. APL Bioeng. 2022. PMID: 35308827 Free PMC article. Review.
References
- Bischoff F.R. and Ponstingl,H. (1991a) Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature, 354, 80–82. - PubMed
- Bischoff F.R. and Görlich,D. (1997) RanBP1 is crucial for the release of RanGTP from importin β-related nuclear transport factors. FEBS Lett., 419, 249–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous