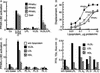Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase - PubMed (original) (raw)
Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase
Ouliana Ziouzenkova et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Increased levels of triglyceride-rich lipoproteins provoke lipid accumulation in the artery wall, triggering early inflammatory responses central to atherosclerosis like endothelial adhesion molecule expression. The endogenous mechanisms limiting such reactions remain poorly defined. Lipoprotein lipase (LPL) plays a central role in lipid metabolism by hydrolyzing triglyceride rich lipoproteins and releasing fatty acids. We found that LPL treatment reversed tumor necrosis factor alpha and very low-density lipoprotein (VLDL)-stimulated endothelial vascular cell adhesion molecule 1 (VCAM1) induction and VCAM1 promoter responses, thus recapitulating effects reported with synthetic peroxisome proliferator-activated receptor (PPAR) agonists. In fact, these LPL effects on VCAM1 were absent in endothelial cells isolated from PPAR alpha-deficient mice. This finding suggests a novel antiinflammatory role for LPL. Further studies reveal specificity for PPAR activation through lipolysis in regards to lipoprotein substrate (VLDL >> LDL > HDL), PPAR isoform (PPAR alpha >> PPAR delta > PPAR gamma), and among fatty acid-releasing lipases. These PPAR responses required intact LPL catalytic activity. In vivo, transgenic mice overexpressing LPL had increased peroxisome proliferation, but not in the genetic absence of PPAR alpha. Although human plasma possesses minimal PPAR alpha activation despite containing abundant free fatty acids, marked PPAR alpha activation is seen with human plasma after LPL is added in vitro or systemically released in vivo. These data suggest a previously uncharacterized pathway in which the key lipolytic enzyme LPL can act on circulating lipoproteins to generate PPAR alpha ligands, providing a potentially important link between lipoprotein metabolism and distal PPAR alpha transcriptional effects.
Figures
Figure 1
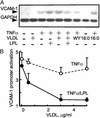
LPL inhibits VLDL/TNFα-mediated VCAM1 expression and promoter activity. (A) Northern analysis of VCAM1 mRNA expression in human EC treated with the PPARα agonist WY14643 (WY, 250 μM); VLDL (5 μg) with or without LPL (200 units/ml, 4 h); saturated FA (16:0) in the presence or absence of TNFα stimulation (50 ng/ml, 17 h). LPL alone had no effect (data not shown). (B) TNFα-mediated VCAM1 promoter (6) activation was tested over a concentration range of VLDL in the presence (solid line) or absence (dashed line) of LPL. All transfection experiments were performed in bovine aortic EC (DMEM, 1% Nutridoma SP). EC were pretreated with VLDL with or without LPL (200 units/ml, 4 h) and then stimulated with TNFα (2 ng/ml, 12 h). The luciferase results were normalized to β-galactosidase activity. For comparison, 250 μM WY14643 suppressed TNFα-induced VCAM1 promoter to a 2.6-fold activation. n = 3, each in triplicate.
Figure 2
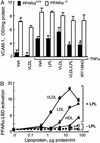
LPL/VLDL inhibits VCAM1 levels through a PPARα-dependent mechanism. (A) VCAM1 surface expression in EC obtained from WT (filled bars) or PPARα−/− (open bars) mice was measured by ELISA as described (6). EC cultured in 96-well plates were treated as in Fig. 1_A_ with murine TNFα (10 ng/ml, 18 h). Data represent absorbance at 410 nm after protein concentration normalization. In WT, but not PPARα−/−, EC both WY14643 and LPL/VLDL significantly decrease VCAM1 levels (*, P < 0.005). Basal VCAM1 levels in PPARα−/− are significantly increased as compared with WT EC (#, P < 0.001). (B) Concentration-dependent activation of PPARα-LBD by various lipoproteins in the presence or absence of LPL (30 units/ml). EC cotransfected with PPARα-LBD, the luciferase response pUASx4-TK-luc, and β-galactosidase constructs were stimulated as shown (18 h, DMEM, 1% delipidated serum). PPARα-LBD activation by fenofibric acid (100 μM) in this experiment was 16.2- ± 1.3-fold.
Figure 3
Lipolysis leads to PPARα activation in a manner selective as to PPAR isoform, lipase, and substrate. (A) PPAR-LBD activation by VLDL in EC was measured in the presence or absence of LPL by using standard pUASx4-TK-luc reporter construct and β-galactosidase normalization approaches as in Fig. 2_B_. Cells were stimulated with LPL (14 h, 30 units/ml) in the presence or absence of VLDL (10 μg/ml). Responses to established PPAR agonists were used for comparison: PPARγ−BRL49653 (BRL, 1 μM); PPARα−WY14643 (WY, 100 μM); PPARδ−carboprostacyclin (carbo, 10 μM). (B) Competition curves were generated by incubating the LPL/VLDL reaction mixture with specific radiolabeled PPAR activators: 5 nM 3H2 compound A with GST-hPPARα (circles) or GST-hPPARγ (triangles), or 2.5 nM 3H2 compound B with GST-hPPARδ (squares). Radioligand displacement in the presence of the indicated concentration of VLDL (0.003–10 μg of protein per ml)/LPL (200 units/ml LPL, 37°C, 1 h) is plotted. Data are from duplicate determinations. K_iS values were obtained from the dose–response curves. (C) PPARα activation occurs through lipolysis mediated by LPL but not some other FA-releasing lipases. EC, transfected as in Fig. 2_B, were treated (18 h) with the lipoproteins shown (10 μg of protein per ml) and either LPL (20 units/ml) or phospholipases PLA2 (20 units/ml), PLD (20 units/ml), or PLC (2 units/ml). Control (open bars), VLDL (filled bars), LDL (horizontally striped bars), and HDL (diagonally striped bars) are shown. (D) The FA generated in Fig. 3_C_ is shown. The FA content in the media was measured spectrophotometrically as described in Methods, SD 5%; bars designated as in Fig. 3_C_. Spectrophotometric determinations correlated with palmitic acid standard in a concentration-dependent manner (data not shown).
Figure 4
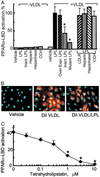
PPARα activation by LPL/VLDL depends on intact LPL catalytic activity. (A) The role of LPL catalytic activity on PPARα-LBD activation was studied in EC by using VLDL (10 μg/ml) combined with various forms of LPL: recombinant LPL (30 units/ml) or heat-inactivated LPL (Inact. LPL); transfected expression constructs for human LPL (Over-expr. LPL) or catalytically inactive mutant LPL. Results are expressed as the percentage of standard LPL/VLDL response. Asterisk indicates a significant decrease in PPARα-LBD activation (P < 0.01, Mann–Whitney test) compared with the appropriate control. No difference was seen in EC overexpressing the LDL receptor (LDLR/LPL) or pretreated with cytochalasin D (10 μM), heparin (100 μg/ml), or heparinase (200 units/ml) for 1 h before LPL/VLDL addition. (B) Labeled VLDL uptake by EC. Cells were stimulated with VLDL (control) and DiI-labeled VLDL (5 μg of protein per ml) with or without LPL (30 units/ml, 18 h). Fluorescent microscopy (×20 magnification) of representative formalin-fixed specimens is shown. DiI VLDL is shown in red and DNA is shown in blue. (C) LPL was preincubated with the lipase inhibitor tetrahydrolipstatin at the concentrations shown (15 min at room temperature) before addition of LPL (30 units/ml)/VLDL (10 μg/ml). Significant inhibition in PPARα-LBD activation (P < 0.01, Mann–Whitney test) as compared with control is shown (*).
Figure 5
Enzymatically intact LPL induces PPARα target gene mRNA expression. (A Left) HepG2 cells were transfected (24 h, DMEM, 1% delipidated serum) with empty vector (PcDNA3), human WT LPL, or a catalytically inactive LPL (mutant LPL), and expression levels were confirmed with RT-PCR (data not shown). Northern analysis reveals that VLDL treatment (5 μg/ml, 3 h) induced ACO mRNA expression in LPL-treated cells, but not if they had been pretreated (15 min) with tetrahydrolipostatin (THL, 10 μM). HepG2 cells expressing the mutant LPL had no ACO induction after VLDL treatment. (Right) Similar Northern results are seen in HEK293 cells transfected and stimulated as before. (B) The concentration response of ACO mRNA expression to VLDL was tested in HepG2 cells incubated with LPL (200 units/ml, 1 h). (C) LPL/VLDL treatment induces ACO and LPL mRNA expression in human Mφ treated with LPL/VLDL as described in Fig. 5_B_. One representative result of three is shown. (D) In vivo analysis of skeletal muscle from WT LPL-overexpressing/PPARα+/+, and LPL-overexpressing/PPARα−/− mice reveals increased peroxisomal proliferation with LPL overexpression in the presence but not absence of PPARα. Peroxisomal expression from fasting mice (age 12 wk, n = 3) was analyzed by using PMP70 antibody.
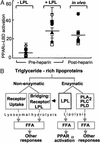
Figure 6
LPL-mediated PPARα activation occurs as a unique component of triglyceride-rich lipoprotein metabolism. (A) PPARα-LBD transactivation assays were performed as in Fig. 2_B_ by using human plasma samples (5%) obtained from six normolipidemic donors at baseline (−LPL), after addition of LPL in vitro (200 units/ml, 37°C, 1 h) to pre-heparin plasma, or after systemic heparin infusion (60 units/kg) in vivo. Symbols represent individual donors; lines indicate mean values. The relative PPARα-LBD fold activation for 10 μM WY14643 in this experiment was 20.3 ± 0.3; FA concentrations were 1.7 ± 0.8 μM (−LPL), 18.2 ± 9.3 μM (+LPL), and 18.2 ± 6.8 μM (post-heparin). (B) The metabolism of TRL occurs through both nonenzymatic and enzymatic mechanisms. Although these pathways ultimately result in FA production and their various downstream effects, PPARα activation through lipolysis seems restricted to some degree to LPL. The data presented suggest that specific mechanisms of endogenous lipolysis can lead to specific transcriptional responses through ligand generation. Thus, LPL-mediated PPARα activation may provide a link between circulating triglyceride-rich lipoproteins and distal PPARα effects such as antiinflammatory responses, FA oxidation, and lipid metabolism. The latter may include a possible positive feedback loop through PPARα leading to increased LPL expression and activity by inducing LPL and repressing ApoCIII.
Similar articles
- Dual roles for lipolysis and oxidation in peroxisome proliferation-activator receptor responses to electronegative low density lipoprotein.
Ziouzenkova O, Asatryan L, Sahady D, Orasanu G, Perrey S, Cutak B, Hassell T, Akiyama TE, Berger JP, Sevanian A, Plutzky J. Ziouzenkova O, et al. J Biol Chem. 2003 Oct 10;278(41):39874-81. doi: 10.1074/jbc.M306786200. Epub 2003 Jul 23. J Biol Chem. 2003. PMID: 12878589 Free PMC article. - VLDL hydrolysis by LPL activates PPAR-alpha through generation of unbound fatty acids.
Ruby MA, Goldenson B, Orasanu G, Johnston TP, Plutzky J, Krauss RM. Ruby MA, et al. J Lipid Res. 2010 Aug;51(8):2275-81. doi: 10.1194/jlr.M005561. Epub 2010 Apr 26. J Lipid Res. 2010. PMID: 20421589 Free PMC article. - Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase.
Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, Yamamoto T, Nakai T. Takahashi S, et al. J Biol Chem. 1995 Jun 30;270(26):15747-54. doi: 10.1074/jbc.270.26.15747. J Biol Chem. 1995. PMID: 7797576 - Postprandial lipoproteins and the molecular regulation of vascular homeostasis.
Botham KM, Wheeler-Jones CP. Botham KM, et al. Prog Lipid Res. 2013 Oct;52(4):446-64. doi: 10.1016/j.plipres.2013.06.001. Epub 2013 Jun 15. Prog Lipid Res. 2013. PMID: 23774609 Review.
Cited by
- Pharmacogenomics of high-density lipoprotein-cholesterol-raising therapies.
Aslibekyan S, Straka RJ, Irvin MR, Claas SA, Arnett DK. Aslibekyan S, et al. Expert Rev Cardiovasc Ther. 2013 Mar;11(3):355-64. doi: 10.1586/erc.12.134. Expert Rev Cardiovasc Ther. 2013. PMID: 23469915 Free PMC article. Review. - Proline- and acidic amino acid-rich basic leucine zipper proteins modulate peroxisome proliferator-activated receptor alpha (PPARalpha) activity.
Gachon F, Leuenberger N, Claudel T, Gos P, Jouffe C, Fleury Olela F, de Mollerat du Jeu X, Wahli W, Schibler U. Gachon F, et al. Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):4794-9. doi: 10.1073/pnas.1002862108. Epub 2011 Mar 7. Proc Natl Acad Sci U S A. 2011. PMID: 21383142 Free PMC article. - Use of fibrates in the metabolic syndrome: A review.
Shipman KE, Strange RC, Ramachandran S. Shipman KE, et al. World J Diabetes. 2016 Mar 10;7(5):74-88. doi: 10.4239/wjd.v7.i5.74. World J Diabetes. 2016. PMID: 26981181 Free PMC article. Review. - Modified methylenedioxyphenol analogs lower LDL cholesterol through induction of LDL receptor expression.
Ying Z, Desikan R, Xu X, Maiseyeu A, Liu C, Sun Q, Ziouzenkova O, Parthasarathy S, Rajagopalan S. Ying Z, et al. J Lipid Res. 2012 May;53(5):879-887. doi: 10.1194/jlr.M022806. Epub 2012 Feb 21. J Lipid Res. 2012. PMID: 22355094 Free PMC article. - Endogenous ligands for nuclear receptors: digging deeper.
Schupp M, Lazar MA. Schupp M, et al. J Biol Chem. 2010 Dec 24;285(52):40409-15. doi: 10.1074/jbc.R110.182451. Epub 2010 Oct 18. J Biol Chem. 2010. PMID: 20956526 Free PMC article. Review.
References
- Glass C K, Witztum J L. Cell. 2001;104:503–516. - PubMed
- Huo Y, Ley K. Acta Physiol Scand. 2001;173:35–43. - PubMed
- Moers A, Fenselau S, Schrezenmeir J. Exp Clin Endocrinol Diabetes. 1997;105:35–37. - PubMed
- Havel R J. Proc Nutr Soc. 1997;56:659–666. - PubMed
- Li H, Cybulsky M I, Gimbrone M A, Jr, Libby P. Arterioscler Thromb. 1993;13:197–204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous