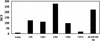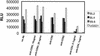Potential role for CD63 in CCR5-mediated human immunodeficiency virus type 1 infection of macrophages - PubMed (original) (raw)
. 2003 Mar;77(6):3624-33.
doi: 10.1128/jvi.77.6.3624-3633.2003.
Daniel Rojo, Kathie Grovit-Ferbas, Christine Yeramian, Cheng Deng, Georges Herbein, Monique R Ferguson, Todd C Pappas, Julie M Decker, Anjali Singh, Ronald G Collman, William A O'Brien
Affiliations
- PMID: 12610138
- PMCID: PMC149503
- DOI: 10.1128/jvi.77.6.3624-3633.2003
Potential role for CD63 in CCR5-mediated human immunodeficiency virus type 1 infection of macrophages
Jana J von Lindern et al. J Virol. 2003 Mar.
Abstract
Macrophages and CD4(+) lymphocytes are the principal target cells for human immunodeficiency virus type 1 (HIV-1) infection, but the molecular details of infection may differ between these cell types. During studies to identify cellular molecules that could be involved in macrophage infection, we observed inhibition of HIV-1 infection of macrophages by monoclonal antibody (MAb) to the tetraspan transmembrane glycoprotein CD63. Pretreatment of primary macrophages with anti-CD63 MAb, but not MAbs to other macrophage cell surface tetraspanins (CD9, CD81, and CD82), was shown to inhibit infection by several R5 and dualtropic strains, but not by X4 isolates. The block to productive infection was postfusion, as assessed by macrophage cell-cell fusion assays, but was prior to reverse transcription, as determined by quantitative PCR assay for new viral DNA formation. The inhibitory effects of anti-CD63 in primary macrophages could not be explained by changes in the levels of CD4, CCR5, or beta-chemokines. Infections of peripheral blood lymphocytes and certain cell lines were unaffected by treatment with anti-CD63, suggesting that the role of CD63 in HIV-1 infection may be specific for macrophages.
Figures
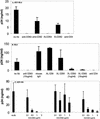
FIG. 1.
Anti-CD63 treatment of primary macrophages inhibits infection by R5 HIV-1 isolates. Cells were preincubated with anti-CD63 (mouse IgG1 isotype, 10 μg/ml) or control antibodies (mouse IgG1, 10 μg/ml) and then infected with the blood-derived R5 isolate HIV-1-BL4 (A) or BL6 (B). For the R5 virus HIV-1-SX (C), anti-CD63, anti-CD82, or anti-CD4 MAb at concentrations ranging from 0 to 3 μg/ml was used. Virus production was measured in culture supernatants at 7 days postinfection by p24 ELISA. In some experiments, cross-linking (XL) of mouse anti-CD63 with a secondary rat anti-mouse MAb was used. Anti-CD4 (Leu3a, 0.5 μg/ml) was used as a positive control, and mouse IgG1, CD9, CD81, and CD82 (all tetraspanin proteins) were used as negative controls (10 μg/ml). Data shown are representative of results with seven different donors and seven different R5 virus isolates.
FIG. 2.
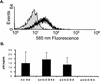
Expression of macrophage surface antigens. Primary macrophages were incubated with the indicated anti-tetraspanin, anti-HLA-DP/DQ/DR, or isotype control MAb for 30 min at 4°C, followed by a 30-min incubation with FITC-labeled secondary antibody. Cells were then fixed and assessed by flow cytometry. Data are representative of results with four different donors. MCF, mean channel fluorescence.
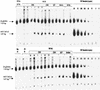
FIG. 3.
Quantitative PCR analysis of new HIV-1 DNA formation. Primary macrophages were pretreated with anti-CD63 (0, 0.1, 1, and 10 μg/ml) or control antibodies, followed by infection with filtered, DNase-treated HIV-1-SX and HIV-1-BaL. After 24 h, cells were lysed and DNA was extracted for PCR amplification with HIV-1 R/U5 primers (lower bands) and with β-globin primers (upper bands) as an internal control. HIV-1 quantitation standards were amplified in parallel. HI, heat-inactivated virus; OKT4A, positive control anti-CD4 MAb (0.5 μg/ml); Medium, no MAb treatment; CD44 and IgG1, negative isotype control MAb (10 μg/ml). Data are representative of five experiments (five donors).
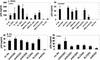
FIG. 4.
Anti-CD63 MAb inhibits infection of macrophages by R5X4 strains, but not X4 strains, of HIV-1. Primary macrophages were incubated with anti-CD63 or negative control MAb (10 μg/ml), anti-CD4 (Leu3A, 0.5 μg/ml), anti-CCR5 (2D7, 10 μg/ml), AMD3100 (10 μg/ml), or a combination of anti-CD63-AMD3100 (5 μg/ml each) or anti-CCR5-AMD3100 (5 μg/ml each) and then infected with the dualtropic strain UG921 or RW92009 (A) or the macrophagetropic X4 strain UG021 or UG024 (B). Following a 7-day incubation, supernatants were harvested for p24 ELISA. Data are representative of four experiments.
FIG. 5.
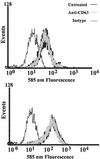
(A) CD63 is expressed on CD4+ lymphocytes. PHA-stimulated CD8-, CD19-, and macrophage-depleted PBL (>90% CD4+) were treated with PE-anti-CD63, FITC-anti-CD4, or matching fluorochrome-conjugated isotype control prior to flow cytometry assessment. The histogram shown is gated on CD4 (FITC)-positive cells. The solid histogram shows the isotype control, and the open histogram shows CD63 expression. Data are representative of results with three donors. (B) Anti-CD63 treatment does not inhibit infection of primary PBL. Cells were treated with anti-CD63 (10 μg/ml) or with control MAb anti-CD9 (10 μg/ml) or anti-CD4 (Leu3A, 0.5 μg/ml) and then infected with HIV-1-SX. Following a 7-day incubation, supernatants were harvested and analyzed for p24 by ELISA. Data are representative of five experiments.
FIG. 6.
Anti-CD63 treatment does not inhibit infection of JC53-BL cells. Cells were treated with anti-CD63 (10 μg/ml), negative control anti-CD82 or anti-CD9 MAb (10 μg/ml), or anti-CD4 (Leu3A, 0.5 μg/ml) and then infected with the primary R5 strains BL2 and BL4, the dualtropic strain 89.6, or the primary X4 virus UG021. Following a 48-h incubation, the cells were lysed and analyzed by luminometry. RLU, relative light units.
FIG. 7.
Anti-CD63 MAb treatment does not affect CD4 or CCR5 expression on macrophages. Cells were pretreated with anti-CD63 MAb and then labeled with an irrelevant PE-isotype control antibody, PE-anti-CD4 (upper graph), or PE-anti-CCR5 (lower graph), followed by flow cytometry assessment.
FIG. 8.
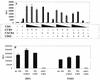
CD63 is not involved in Env-mediated cell-cell fusion. (A) Cell-cell fusion in QT6 cells. The indicated expression constructs were transfected into QT6 cells and allowed to fuse with QT6 cells expressing ADA Env (striped bars) or HXB Env (black bars). Relative levels of fusion were determined by luciferase production at 6 h, as described in Materials and Methods. Decreasing amounts of transfected CD4 plasmid are shown by decrescendo bars. (B) Macrophage fusion assay. Macrophages were treated with anti-CD63 or control MAb followed by cross-linking (XL) with anti-mouse secondary antibody, and the relative levels of fusion with JRFL or UG021 Env-transfected 293T cells were measured at 6 h, as described in Materials and Methods. RLU, relative light units.
Similar articles
- CD63 is not required for production of infectious human immunodeficiency virus type 1 in human macrophages.
Ruiz-Mateos E, Pelchen-Matthews A, Deneka M, Marsh M. Ruiz-Mateos E, et al. J Virol. 2008 May;82(10):4751-61. doi: 10.1128/JVI.02320-07. Epub 2008 Mar 5. J Virol. 2008. PMID: 18321974 Free PMC article. - Recombinant extracellular domains of tetraspanin proteins are potent inhibitors of the infection of macrophages by human immunodeficiency virus type 1.
Ho SH, Martin F, Higginbottom A, Partridge LJ, Parthasarathy V, Moseley GW, Lopez P, Cheng-Mayer C, Monk PN. Ho SH, et al. J Virol. 2006 Jul;80(13):6487-96. doi: 10.1128/JVI.02539-05. J Virol. 2006. PMID: 16775336 Free PMC article. - HIV-1 R5 Macrophage-Tropic Envelope Glycoprotein Trimers Bind CD4 with High Affinity, while the CD4 Binding Site on Non-macrophage-tropic, T-Tropic R5 Envelopes Is Occluded.
Quitadamo B, Peters PJ, Repik A, O'Connell O, Mou Z, Koch M, Somasundaran M, Brody R, Luzuriaga K, Wallace A, Wang S, Lu S, McCauley S, Luban J, Duenas-Decamp M, Gonzalez-Perez MP, Clapham PR. Quitadamo B, et al. J Virol. 2018 Jan 2;92(2):e00841-17. doi: 10.1128/JVI.00841-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29118121 Free PMC article. - Biological parameters of HIV-1 infection in primary intestinal lymphocytes and macrophages.
Smith PD, Meng G, Sellers MT, Rogers TS, Shaw GM. Smith PD, et al. J Leukoc Biol. 2000 Sep;68(3):360-5. J Leukoc Biol. 2000. PMID: 10985252 Review. - Tetraspanin functions during HIV-1 and influenza virus replication.
Thali M. Thali M. Biochem Soc Trans. 2011 Apr;39(2):529-31. doi: 10.1042/BST0390529. Biochem Soc Trans. 2011. PMID: 21428933 Free PMC article. Review.
Cited by
- A post-entry role for CD63 in early HIV-1 replication.
Li G, Dziuba N, Friedrich B, Murray JL, Ferguson MR. Li G, et al. Virology. 2011 Apr 10;412(2):315-24. doi: 10.1016/j.virol.2011.01.017. Epub 2011 Feb 26. Virology. 2011. PMID: 21315401 Free PMC article. - WFDC1/ps20 is a novel innate immunomodulatory signature protein of human immunodeficiency virus (HIV)-permissive CD4+ CD45RO+ memory T cells that promotes infection by upregulating CD54 integrin expression and is elevated in HIV type 1 infection.
Alvarez R, Reading J, King DF, Hayes M, Easterbrook P, Farzaneh F, Ressler S, Yang F, Rowley D, Vyakarnam A. Alvarez R, et al. J Virol. 2008 Jan;82(1):471-86. doi: 10.1128/JVI.00939-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942534 Free PMC article. - CD63 is not required for production of infectious human immunodeficiency virus type 1 in human macrophages.
Ruiz-Mateos E, Pelchen-Matthews A, Deneka M, Marsh M. Ruiz-Mateos E, et al. J Virol. 2008 May;82(10):4751-61. doi: 10.1128/JVI.02320-07. Epub 2008 Mar 5. J Virol. 2008. PMID: 18321974 Free PMC article. - Targeting of tetraspanin proteins--potential benefits and strategies.
Hemler ME. Hemler ME. Nat Rev Drug Discov. 2008 Sep;7(9):747-58. doi: 10.1038/nrd2659. Nat Rev Drug Discov. 2008. PMID: 18758472 Free PMC article. Review. - Tetraspanin CD63 is a regulator of HIV-1 replication.
Fu E, Pan L, Xie Y, Mu D, Liu W, Jin F, Bai X. Fu E, et al. Int J Clin Exp Pathol. 2015 Feb 1;8(2):1184-98. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 25973004 Free PMC article.
References
- Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. - PubMed
- Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. - PMC - PubMed
- Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL088999-05/HL/NHLBI NIH HHS/United States
- R01 HL088999/HL/NHLBI NIH HHS/United States
- AI52041/AI/NIAID NIH HHS/United States
- NS27405/NS/NINDS NIH HHS/United States
- R01 AI035502/AI/NIAID NIH HHS/United States
- R56 AI052041/AI/NIAID NIH HHS/United States
- AI46250/AI/NIAID NIH HHS/United States
- P01 NS027405/NS/NINDS NIH HHS/United States
- AI35502/AI/NIAID NIH HHS/United States
- T32 AI007388/AI/NIAID NIH HHS/United States
- T32/AI07388/AI/NIAID NIH HHS/United States
- R01 AI052041/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous