De novo formation, fusion and fission of mammalian COPII-coated endoplasmic reticulum exit sites - PubMed (original) (raw)
De novo formation, fusion and fission of mammalian COPII-coated endoplasmic reticulum exit sites
David J Stephens. EMBO Rep. 2003 Feb.
Abstract
Transport between the endoplasmic reticulum (ER) and Golgi is mediated by the sequential action of the COPII and COPI coat complexes. COPII subunits are recruited to the ER membrane where they mediate the selection of cargo for transport to the Golgi, and also membrane deformation and vesicle formation. New ER exit sites can be generated by lateral growth and medial fission (in Pythium sp.) or by de novo formation (in Pichia pastoris) but it is not known how mammalian ER exit sites form. Here, time-lapse imaging of COPII-coated structures in live mammalian cells reveals that the number of ER export sites increases greatly during interphase by de novo formation. These results show the fusion of pre-existing ER export sites and the fission of larger structures. These three mechanisms of de novo formation, fusion and fission probably cooperate to regulate the size of these sites in mammalian cells.
Figures
Figure 1
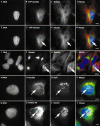
COPII-coated ER export sites greatly increase in number through interphase. Live cell imaging of YFP–Sec23A during interphase shows a marked increase in the number of COPII-coated ER exit sites. (A) Numbered frames correspond to minutes after the start of imaging. Images were acquired at 1 min intervals. The asterisk at 500 min of imaging denotes the migration of fluorescent structures from the juxtanuclear area to a region above the nucleus. (B) Enlarged view of the same cells as in (A) at the start and after 240 min of imaging. Scale bar, 5 μm. See Supplementary Information, which clearly shows the translocation of these structures to the juxtanuclear area at a very low rate (∼5–15 μm h−1).
Figure 2
Relocation of YFP–Sec23A to an area above the nucleus is correlated with changes in the microtubule network and is accompanied by a relocation of Golgi matrix and membranes. (A–C, E–G) Images of the same cell at different focal planes, showing DNA, YFP–Sec23A and tubulin, as indicated. (I–K) DNA with giantin and tubulin immunofluorescence; (M–O) DNA, Sec23A and galactosyltransferase (GalT) immunofluorescence; (Q–S) DNA, ERGIC-53 and giantin immunofluorescence, as indicated. (D, H, L, P, T) Merged images of the preceding three panels in each case. Scale bars, 5 μm except for (I–L), where scale bar represents 15 μm.
Figure 3
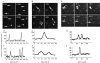
De novo formation, fission and fusion of COPII-coated ER export sites. (A–C) De novo formation of COPII-coated ER exit sites. (A) Frames are each separated by 1 min. Arrows highlight pre-existing structures; the arrowhead marks a newly appearing structure (appearing at +1 min). The asterisk marks a structure that seems to divide in the last minute of imaging (compare +1 min with +2 min). The dimensions of the region shown are 10.7 μm × 10.7 μm. (B, C) Plots of fluorescence intensity along a line drawn through and connecting the structures marked with arrows in (A), 0 min and +3 min respectively. (D–F) Fission of COPII-coated ER exit sites. A single, bright exit site (arrow) is seen to generate two discrete sites (+20 s); a further new site is seen adjacent to one of these (+40 s). The dimensions of the region shown are 8.1 μm × 8.1 μm. (E, F) Plots of fluorescence intensity across a diagonal line (passing through the single structure in (D), 0 s, and both structures in (D), +40 s). (G–I) Fusion of COPII-coated ER exit sites. Two COPII-coated structures are shown (arrowheads, 0–120 s) that undergo fusion and persist as a single structure (arrow, 130–200 s). The dimensions of the region shown are 11.4 μm × 11.4 μm. (H, I) Intensity profiles through the structures highlighted in (G), 0 s, and (G), 130 s, showing that the two structures seen at t = 0 s fuse to form one.
Figure 4
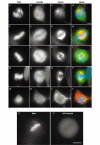
New ER export sites are functional in ER-to-Golgi transport. YFP–Sec23A-coated ER export sites co-localize with ERGIC-53 throughout interphase. (A, B) Early interphase; (C, D) late interphase (note the increased number of exit sites compared with (A) and (B), and the location of ER exit sites above the nucleus (asterisk)). (A, C) YFP–Sec23A; (B, D) anti-ERGIC-53 immunofluorescence. Arrowheads mark a selection of co-localizing structures. Scale bars, 5 μm.
Figure 5
YFP–Sec23A is transiently redistributed to the cytosol during mitosis. Distribution of Sec23A during mitosis: prophase/prometaphase (A–D); metaphase (E–H); metaphase (I–L); early anaphase (M–P); telophase (Q–T) (note midbody stained with α-tubulin linking to that adjoining daughter cell). (U, V) Imaging of a living cell at mitosis reveals cytosolic localization of YFP–Sec23A during metaphase. (A, E, I, M, Q) DAPI staining; (B, F, J, N, R) Sec23A immunostaining; (C, G, K, O, S) α-tubulin staining; (D, H, L, P, T) merged images of the three preceding panels in each case. (U) DAPI image showing chromosomes aligned at metaphase; (V) YFP–Sec23A fluorescence showing diffuse labelling throughout the cell. Note that punctate Sec23A staining is seen throughout mitosis but the predominant localization is cytosolic, especially when chromosomes are aligned at metaphase. Scale bars, 5 μm.
Similar articles
- COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers.
Shomron O, Nevo-Yassaf I, Aviad T, Yaffe Y, Zahavi EE, Dukhovny A, Perlson E, Brodsky I, Yeheskel A, Pasmanik-Chor M, Mironov A, Beznoussenko GV, Mironov AA, Sklan EH, Patterson GH, Yonemura Y, Sannai M, Kaether C, Hirschberg K. Shomron O, et al. J Cell Biol. 2021 Jun 7;220(6):e201907224. doi: 10.1083/jcb.201907224. J Cell Biol. 2021. PMID: 33852719 Free PMC article. - COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites.
Stephens DJ, Lin-Marq N, Pagano A, Pepperkok R, Paccaud JP. Stephens DJ, et al. J Cell Sci. 2000 Jun;113 ( Pt 12):2177-85. doi: 10.1242/jcs.113.12.2177. J Cell Sci. 2000. PMID: 10825291 - Vesicle-mediated export from the ER: COPII coat function and regulation.
D'Arcangelo JG, Stahmer KR, Miller EA. D'Arcangelo JG, et al. Biochim Biophys Acta. 2013 Nov;1833(11):2464-72. doi: 10.1016/j.bbamcr.2013.02.003. Epub 2013 Feb 15. Biochim Biophys Acta. 2013. PMID: 23419775 Free PMC article. Review. - Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites.
Yang YD, Elamawi R, Bubeck J, Pepperkok R, Ritzenthaler C, Robinson DG. Yang YD, et al. Plant Cell. 2005 May;17(5):1513-31. doi: 10.1105/tpc.104.026757. Epub 2005 Apr 1. Plant Cell. 2005. PMID: 15805489 Free PMC article. - COPII and selective export from the endoplasmic reticulum.
Barlowe C. Barlowe C. Biochim Biophys Acta. 1998 Aug 14;1404(1-2):67-76. doi: 10.1016/s0167-4889(98)00047-0. Biochim Biophys Acta. 1998. PMID: 9714742 Review.
Cited by
- Vesicular and uncoated Rab1-dependent cargo carriers facilitate ER to Golgi transport.
Westrate LM, Hoyer MJ, Nash MJ, Voeltz GK. Westrate LM, et al. J Cell Sci. 2020 Jul 24;133(14):jcs239814. doi: 10.1242/jcs.239814. J Cell Sci. 2020. PMID: 32616562 Free PMC article. - Assembly, organization, and function of the COPII coat.
Hughes H, Stephens DJ. Hughes H, et al. Histochem Cell Biol. 2008 Feb;129(2):129-51. doi: 10.1007/s00418-007-0363-x. Epub 2007 Dec 4. Histochem Cell Biol. 2008. PMID: 18060556 Free PMC article. Review. - Misplaced Golgi Elements Produce Randomly Oriented Microtubules and Aberrant Cortical Arrays of Microtubules in Dystrophic Skeletal Muscle Fibers.
Oddoux S, Randazzo D, Kenea A, Alonso B, Zaal KJM, Ralston E. Oddoux S, et al. Front Cell Dev Biol. 2019 Sep 18;7:176. doi: 10.3389/fcell.2019.00176. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31620435 Free PMC article. - Mechanisms regulating the sorting of soluble lysosomal proteins.
Meraş İ, Maes J, Lefrancois S. Meraş İ, et al. Biosci Rep. 2022 May 27;42(5):BSR20211856. doi: 10.1042/BSR20211856. Biosci Rep. 2022. PMID: 35394021 Free PMC article. Review. - Cdc2 kinase-dependent disassembly of endoplasmic reticulum (ER) exit sites inhibits ER-to-Golgi vesicular transport during mitosis.
Kano F, Tanaka AR, Yamauchi S, Kondo H, Murata M. Kano F, et al. Mol Biol Cell. 2004 Sep;15(9):4289-98. doi: 10.1091/mbc.e03-11-0822. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254263 Free PMC article.
References
- Aridor M., Bannykh S.I., Rowe T. & Balch W.E. (1999) Cargo can modulate COPII vesicle formation from the endoplasmic reticulum. J. Biol. Chem., 274, 4389–4399. - PubMed
- Barlowe C. (2002) COPII-dependent transport from the endoplasmic reticulum. Curr. Opin. Cell Biol., 14, 417–422. - PubMed
- Barlowe C. et al. . (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell, 77, 895–907. - PubMed
- Bevis B.J., Hammond A.T., Reinke C.A. & Glick B.S. (2002) De novo formation of transitional ER sites and Golgi structures in Pichia pastoris. Nature Cell Biol., 4, 750–756. - PubMed
- Bracker C.E., Morré D.J. & Grove S.N. (1996) Structure, differentiation and multiplication of the Golgi apparatus in fungal hyphae. Protoplasma, 194, 250–274.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources