Implications of a RAD54L polymorphism (2290C/T) in human meningiomas as a risk factor and/or a genetic marker - PubMed (original) (raw)
Implications of a RAD54L polymorphism (2290C/T) in human meningiomas as a risk factor and/or a genetic marker
Paola E Leone et al. BMC Cancer. 2003.
Abstract
Background: RAD54L (OMIM 603615, Locus Link 8438) has been proposed as a candidate oncosupressor in tumours bearing a non-random deletion of 1p32, such as breast or colon carcinomas, lymphomas and meningiomas. In a search for RAD54L mutations in 29 menigiomas with allelic deletions in 1p, the only genetic change observed was a silent C/T transition at nucleotide 2290 in exon 18. In this communication the possible association of the 2290C/T polymorphism with the risk of meningiomas was examined. In addition, the usefulness of this polymorphism as a genetic marker within the meningioma consensus deletion region in 1p32 was also verified. The present study comprises 287 blood control samples and 70 meningiomas from Spain and Ecuador. Matched blood samples were only available from Spanish patients.
Results: The frequency of the rare allele-T and heterozygotes for the 2290C/T polymorphism in the blood of Spanish meningioma patients and in the Ecuadorian meningioma tumours was higher than in the control population (P < 0.05). Four other rare variants (2290C/G, 2299C/G, 2313G/A, 2344A/G) were found within 50 bp at the 3' end of RAD54L. Frequent loss of heterozygosity for the 2290C/T SNP in meningiomas allowed to further narrow the 1p32 consensus region of deletion in meningiomas to either 2.08 Mbp - within D1S2713 (44.35 Mbp) and RAD54L (46.43 Mbp) - or to 1.47 Mbp - within RAD54L and D1S2134 (47.90 Mbp) - according to recent gene mapping results.
Conclusion: The statistical analysis of genotypes at the 2290C/T polymorphism suggest an association between the rare T allele and the development of meningeal tumours. This polymorphism can be used as a genetic marker inside the consensus deletion region at 1p32 in meningiomas.
Figures
Figure 1
Status of the 2290C/T polymorphism in meningiomas from Ecuador. DNA was extracted from paraffin archival and 2290 C/T alleles were characterised by PCR-SSCP as described in methods. The figure shows a representative SSCP of 37 cases. Arrowhead point to T and C alleles migration in the gel.
Figure 2
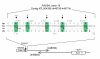
Variants and mismatches in exon 18 of RAD54L and molecular linkage of RAD54L and MUF1 through an inverse overlapping of untranslated mRNA. In the upper side of the figure, variants found in meningiomas (numbered 1–2, 3, 4, 5 and 6) and mismatches reported in the NCBI (Evidence Viewer, graphic display for RAD54L) (1, 4 and 5) are shown in bold within green boxes. The figure displays from top to bottom: the reference protein (Rp) and mRNA (Rs) at the 3' end of RAD54L, the variants and mismatches (Vr) and their corresponding protein sequences (Vp). In the lower part of the figure the inverse overlapping 70 bp sequence shared by RAD54L and MUF1 3'UTR is shown. Figures in brackets correspond to nucleotide positions in the NT_004386 contig.
Figure 3
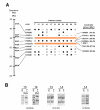
Summary of LOH analysis and map information. A. Sequence maps of 1p markers and genes were obtained from NCBI gene maps and UniSTS records (updated as of September 2002). Salmon coloured horizontal lines delimit the smallest overlapping region flanked by D1S2713 and D1S2134 [6]. LOH results for 11 RAD54L polymorphism informative cases are taken from previous published and unpublished results (see the text). Open and closed circles correspond to retention and loss of heterozygosity respectively. The horizontal bar represents uninformative constitutional homozygosis. Patient identification as in ref. 5. B. SSCP of constitutive (C) and tumoral (T) DNA from patients 13 and 14, showing LOH of RAD54L and retention of heterozygosity for D1S197 and D1S232 markers.
Similar articles
- Search for mutations of the hRAD54 gene in sporadic meningiomas with deletion at 1p32.
Mendiola M, Bello MJ, Alonso J, Leone PE, Vaquero J, Sarasa JL, Kusak ME, De Campos JM, Pestaña A, Rey JA. Mendiola M, et al. Mol Carcinog. 1999 Apr;24(4):300-4. doi: 10.1002/(sici)1098-2744(199904)24:4<300::aid-mc8>3.0.co;2-g. Mol Carcinog. 1999. PMID: 10326867 - Identification of a consistent region of allelic loss on 1p32 in meningiomas: correlation with increased morbidity.
Sulman EP, Dumanski JP, White PS, Zhao H, Maris JM, Mathiesen T, Bruder C, Cnaan A, Brodeur GM. Sulman EP, et al. Cancer Res. 1998 Aug 1;58(15):3226-30. Cancer Res. 1998. PMID: 9699646 - Association of MTHFR, MTRR and RAD54L Gene Variations with Meningioma and Correlation with Tumor's Histopathological Characteristics on Turkish Cohort.
Avsar T, Mohiyuddin R, Calis S, Yapicier O, Kilic T. Avsar T, et al. Turk Neurosurg. 2021;31(4):587-593. doi: 10.5137/1019-5149.JTN.33347-20.2. Turk Neurosurg. 2021. PMID: 34169999 - Molecular analysis of alterations of the p18INK4c gene in human meningiomas.
Santarius T, Kirsch M, Nikas DC, Imitola J, Black PM. Santarius T, et al. Neuropathol Appl Neurobiol. 2000 Feb;26(1):67-75. doi: 10.1046/j.1365-2990.2000.00219.x. Neuropathol Appl Neurobiol. 2000. PMID: 10736068 - The role of folate metabolism-related gene polymorphisms in the development of meningiomas.
Drakos SS, Anifantaki F, Zarros A, Liapi C. Drakos SS, et al. Cancer Genomics Proteomics. 2010 Mar-Apr;7(2):105-9. Cancer Genomics Proteomics. 2010. PMID: 20335525 Review.
Cited by
- Sequence Neighborhoods Enable Reliable Prediction of Pathogenic Mutations in Cancer Genomes.
Banerjee S, Raman K, Ravindran B. Banerjee S, et al. Cancers (Basel). 2021 May 14;13(10):2366. doi: 10.3390/cancers13102366. Cancers (Basel). 2021. PMID: 34068918 Free PMC article. - Early recurrences in histologically benign/grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone.
Maillo A, Orfao A, Espinosa AB, Sayagués JM, Merino M, Sousa P, Lara M, Tabernero MD. Maillo A, et al. Neuro Oncol. 2007 Oct;9(4):438-46. doi: 10.1215/15228517-2007-026. Epub 2007 Aug 17. Neuro Oncol. 2007. PMID: 17704362 Free PMC article. - Significant effect of homologous recombination DNA repair gene polymorphisms on pancreatic cancer survival.
Li D, Liu H, Jiao L, Chang DZ, Beinart G, Wolff RA, Evans DB, Hassan MM, Abbruzzese JL. Li D, et al. Cancer Res. 2006 Mar 15;66(6):3323-30. doi: 10.1158/0008-5472.CAN-05-3032. Cancer Res. 2006. PMID: 16540687 Free PMC article. - Genetics and genomic medicine in Ecuador.
Paz-Y-Miño C, Guillen Sacoto MJ, Leone PE. Paz-Y-Miño C, et al. Mol Genet Genomic Med. 2015 Dec 21;4(1):9-17. doi: 10.1002/mgg3.192. eCollection 2016 Jan. Mol Genet Genomic Med. 2015. PMID: 26788533 Free PMC article. - The Curcumin Analog PAC Is a Potential Solution for the Treatment of Triple-Negative Breast Cancer by Modulating the Gene Expression of DNA Repair Pathways.
Almalki E, Al-Amri A, Alrashed R, Al-Zharani M, Semlali A. Almalki E, et al. Int J Mol Sci. 2023 Jun 2;24(11):9649. doi: 10.3390/ijms24119649. Int J Mol Sci. 2023. PMID: 37298600 Free PMC article.
References
- Bondy M, Lignon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29:197–205. - PubMed
- Kuratsu J, Kochi M, Ushio Y. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. 2000;92:766–770. - PubMed
- Harada T, Irving RM, Xuereb JH, Barton DE, Hardy DG, Moffat DA, Maher ER. Molecular genetic investigation of the neurofibromatosis type 2 tumor suppressor gene in sporadic meningioma. J Neurosurg. 1996;84:847–851. - PubMed
- Lamszus K, Vahldiek F, Mautner VF, Schichor C, Tonn J, Stavron D, Fillbrandt , Westphal M, Kluwe L. Allelic loses in neurofibromatosis 2-associated meningiomas. J Neuropathol Exp Neurol. 2000;59:504–512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous