Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates - PubMed (original) (raw)
Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates
J W Hooper et al. Virology. 2003.
Abstract
Two major infectious forms of vaccinia virus (VACV) have been described: the intracellular mature virion (IMV), and the extracellular enveloped virion (EEV). Due to their stability in the environment, IMVs play a predominant role in host-to-host transmission, whereas EEVs play an important role in dissemination within the host. In a previous report, we demonstrated that mice vaccinated with VACV L1R (IMV immunogen) and A33R (EEV immunogen) were protected from a lethal poxvirus challenge. Vaccination with a combination of both genes conferred greater protection than either gene alone, suggesting that an immune response against both IMV and EEV is advantageous. Here, we report that in mice individually administered DNA vaccines with two different VACV immunogens, A27L (IMV immunogen) or B5R (EEV immunogen), failed to significantly protect; however, vaccination with a combination of both genes conferred a high level of protection. Mice were completely protected when vaccinated with a combination of four VACV genes (A27L + A33R + L1R + B5R). Rhesus macaques vaccinated with this four-gene-combination developed appropriate antibody responses to each protein. Antibody responses elicited by this vaccine cross-reacted with monkeypox virus orthologous proteins. These data indicate that a gene-based vaccine comprised of the VACV A27L + A33R + L1R + B5R genes may be a useful candidate to protect against other orthopoxviruses, including those that cause monkeypox and smallpox.
Figures
Fig. 1
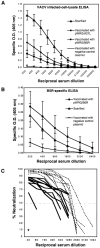
Antibody responses elicited by gene gun vaccination with pWRG/A27L or pWRG/B5R. Sera from vaccinated mice were tested for vaccinia-specific antibodies using (A) vaccinia-infected-cell-lysate ELISA, and (B) B5R-specific ELISA. The mean O.D. values and standard deviations for the nine mice in each group are shown. (C) PRNT were performed on sera from mice vaccinated with pWRG/A27L (solid lines) or scarified (dashed lines) to evaluate the vaccinia-neutralizing antibody response. Representative serum samples diluted 1:40 from mice vaccinated with a negative control plasmid are shown as symbols.
Fig. 2
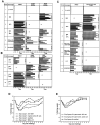
Protection experiments in mice. (A–C) Mice were vaccinated with a DNA vaccine containing the indicated immunogen(s) or scarified with live VACV-CONN. Sera were collected after the final vaccination and the animals were challenged i.p. with 5 × 108 PFU of VACV-WR. The prechallenge sera were evaluated for NAbs by PRNT, and for anti-B5R or anti-A33R antibodies by protein-specific ELISA. In panel C an infected-cell-lysate ELISA was used to measure anti-A27L and anti-B5R responses. PRNT and ELISA end-point titers for individual mice in each group are shown as bars. Filled bars represent animals that did not survive challenge. Numbers to the right of filled bars indicate the day of death. In groups where positive antibody responses were detected, geometric mean titers (GMT) are shown. NT, not tested. (D–E) For groups where all mice were protected, weight loss was used as a measure of relative animal health. The average % of starting weight at times after challenge is shown. Panels A, B, and D contain data from challenge Experiment 2; panels C and E contain data from challenge Experiment 3.
Fig. 3
Candidate DNA vaccine elicits antibody responses against IMV and EEV in nonhuman primates. (A) Monkeys were vaccinated with a four-gene-combination, L1R alone, live VACV by the s. c. route, or by scarification. PRNT were performed to evaluate the NAb response. The last dilution reducing plaque number by either 50% or 80% are shown. Vaccinia-infected-cell lysate ELISA and vaccinia-purified virion ELISA were performed and the end-point titers were determined. Anti-B5R and anti-A33R immunostaining assays were performed to determine the highest dilution of sera resulting in positive protein-specific staining. Open bars were not expected to be positive and served as negative controls. For samples that were negative by immunostaining but suspected to be positive, we performed RIPA. A + symbol above a bar indicates this sample was positive by RIPA, a +/− symbol indicates a weak band was detected by RIPA, and a ? indicates the sample is negative by RIPA. (B) Representative RIPA data using sera from two monkeys vaccinated s.c. with the four-gene-combination (RC114 and CH93), with live VACV (CH21 and AA016), or with live VACV by Dryvax scarification (CH86 and CH39) are shown. Each lane represents a RIPA involving the indicated monkey serum and antigen in the form of cell lysate from cells transfected with a plasmid expressing either L1R, A33R, B5R, A27L, or a control plasmid with no insert (−); or cell lysate from COS cells infected with VACV-WR. A serum from a naive monkey (normal sera) served as a negative control. L1R was not detected using these sera. (C) Sera from monkeys vaccinated with L1R alone (CH63, CH74, and CH79) contained antibodies that immunoprecipitated L1R. L1R-specific MAb-7D11 served as a positive control. A + indicates immunoprecipitation from cells transfected with pWRG/L1R, and − indicates immunoprecipitation from cells transfected with a control plasmid with no insert, pWRG7077. (D) Serum from a monkey vaccinated with L1R (CH63), or a negative control (CH85), or L1R-specific MAb-7D11, or a negative control MAb-3d7 were tested by RIPA using VACV-WR infected-cell lysate as the source of antigen. A + indicates immunoprecipitation from cells infected with VACV-WR, and − indicates immunoprecipitation from mock infected cells. Molecular mass markers (M) are shown in kDa on the left and the position of specific vaccinia immunogens are shown on right.
Fig. 4
Comparison of VACV, MPOV, and VARV A27L; A33R, L1R, and B5R orthologs. The amino acid sequences of the proteins encoded by our DNA vaccine plasmids (VACV-CONN) were compared with VACV-WR, MPOV-Z79, MPOV-Z96, VARV-IND, and VARV-GAR orthologs. Amino acids that were identical in all viruses are boxed in black. Positions where variation occurs are either shown boxed in gray (conserved substitutions), white (nonconserved substitutions), or as a dash (deletion). Numbers at left are amino acid position. MPOV-Z79, MPOV- Z96, VACV-CONN, VACV-WR, VARV-IND, and VARV-GAR A27L ortholog accession numbers are submitted AY160186, NP_536566, AY160184, P11258, NP_042178, and D72167, respectively; A33R ortholog accession numbers are AY160188, NP_536572, AAF63733, BAA01805, CAA47507, and B72168, respectively; L1R ortholog accession numbers are submitted AY160187, NP_536507, AAF63732, P07612, NP_042117, and 672159, respectively; B5R ortholog accession numbers are submitted AY160189, NP_536594, submitted AY160185, Q01227, NP_042219, and E72150, respectively.
Fig. 5
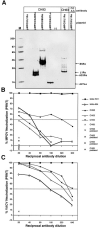
Antibodies elicited by DNA vaccination with vaccinia-based plasmids react with monkeypox virus orthologous proteins. (A) COS cells were transfected with DNA vaccine plasmids containing the monkeypox virus L1R, A33R, B5R, A27L ortholog, or empty vector; pMPOX/L1Ro, pMPOX/A33Ro, pMPOX/B5Ro, pMPOX/A27Lo, (−), respectively. RIPA were performed using serum from a monkey, CH93, vaccinated with the four-gene-combination (plasmids, pWRG/L1R, pWRG/A33R, pWRG/B5R, and pWRG/A27L). Serum from a monkey vaccinated with pWRG/L1R alone, CH63, was used to immunoprecipitate monkeypox virus L1R ortholog. A no antibody (no Ab) control was included in the monkeypox virus L1R ortholog RIPA. Molecular mass markers (M) are shown in kDa on the left and the position of specific vaccinia immunogens are shown on right. The o (e.g., L1R_o_) indicates ortholog. PRNT were performed to detect (B) MPOV-neutralizing antibodies or (C) vaccinia-neutralizing antibodies in the sera from DNA vaccinated monkeys or relevant MAbs. Serum samples collected before vaccination (prebleeds) were also tested.
Similar articles
- Parainfluenza virus 5-based vaccine vectors expressing vaccinia virus (VACV) antigens provide long-term protection in mice from lethal intranasal VACV challenge.
Clark KM, Johnson JB, Kock ND, Mizel SB, Parks GD. Clark KM, et al. Virology. 2011 Oct 25;419(2):97-106. doi: 10.1016/j.virol.2011.08.005. Epub 2011 Aug 31. Virology. 2011. PMID: 21885079 Free PMC article. - Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox.
Hooper JW, Thompson E, Wilhelmsen C, Zimmerman M, Ichou MA, Steffen SE, Schmaljohn CS, Schmaljohn AL, Jahrling PB. Hooper JW, et al. J Virol. 2004 May;78(9):4433-43. doi: 10.1128/jvi.78.9.4433-4443.2004. J Virol. 2004. PMID: 15078924 Free PMC article. - Differential antigen requirements for protection against systemic and intranasal vaccinia virus challenges in mice.
Kaufman DR, Goudsmit J, Holterman L, Ewald BA, Denholtz M, Devoy C, Giri A, Grandpre LE, Heraud JM, Franchini G, Seaman MS, Havenga MJ, Barouch DH. Kaufman DR, et al. J Virol. 2008 Jul;82(14):6829-37. doi: 10.1128/JVI.00353-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448519 Free PMC article. - DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge.
Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. Hooper JW, et al. Virology. 2000 Jan 20;266(2):329-39. doi: 10.1006/viro.1999.0096. Virology. 2000. PMID: 10639319 - Modulating Vaccinia Virus Immunomodulators to Improve Immunological Memory.
Albarnaz JD, Torres AA, Smith GL. Albarnaz JD, et al. Viruses. 2018 Feb 28;10(3):101. doi: 10.3390/v10030101. Viruses. 2018. PMID: 29495547 Free PMC article. Review.
Cited by
- Recombinant Sheep Pox Virus Proteins Elicit Neutralizing Antibodies.
Chervyakova OV, Zaitsev VL, Iskakov BK, Tailakova ET, Strochkov VM, Sultankulova KT, Sandybayev NT, Stanbekova GE, Beisenov DK, Abduraimov YO, Mambetaliyev M, Sansyzbay AR, Kovalskaya NY, Nemchinov LG, Hammond RW. Chervyakova OV, et al. Viruses. 2016 Jun 7;8(6):159. doi: 10.3390/v8060159. Viruses. 2016. PMID: 27338444 Free PMC article. - Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection.
Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, Kirk J, Eppler B, Klinman DM, Sui Y, Gagnon S, Belyakov IM, Mumper RJ, Berzofsky JA. Zhu Q, et al. Nat Med. 2012 Aug;18(8):1291-6. doi: 10.1038/nm.2866. Epub 2012 Jul 15. Nat Med. 2012. PMID: 22797811 Free PMC article. - The 1.51-Angstrom structure of the poxvirus L1 protein, a target of potent neutralizing antibodies.
Su HP, Garman SC, Allison TJ, Fogg C, Moss B, Garboczi DN. Su HP, et al. Proc Natl Acad Sci U S A. 2005 Mar 22;102(12):4240-5. doi: 10.1073/pnas.0501103102. Epub 2005 Mar 10. Proc Natl Acad Sci U S A. 2005. PMID: 15761054 Free PMC article. - Structural basis for the binding of the neutralizing antibody, 7D11, to the poxvirus L1 protein.
Su HP, Golden JW, Gittis AG, Hooper JW, Garboczi DN. Su HP, et al. Virology. 2007 Nov 25;368(2):331-41. doi: 10.1016/j.virol.2007.06.042. Epub 2007 Aug 3. Virology. 2007. PMID: 17688903 Free PMC article. - Protection against lethal vaccinia virus challenge by using an attenuated matrix protein mutant vesicular stomatitis virus vaccine vector expressing poxvirus antigens.
Braxton CL, Puckett SH, Mizel SB, Lyles DS. Braxton CL, et al. J Virol. 2010 Apr;84(7):3552-61. doi: 10.1128/JVI.01572-09. Epub 2010 Jan 20. J Virol. 2010. PMID: 20089648 Free PMC article.
References
- Boulter E.A., Zwartouw H.T., Titmuss H.J., Maber H.B. The nature of the immune state produced by inactivated vaccinia virus in rabbits. Am. J. Epidemiol. 1971;94:612–620. - PubMed
- Chertov O.Y.u, Telezhinskaya I.N., Zaitseva E.V., Golubeva T.B., Zinov’ev V.V., Ovechkina L.G., Mazkova L.B., Malygin E.G. Amino acid sequence determination of vaccinia virus immunodominant protein p35 and identification of the gene. Biomed. Sci. 1991;2:151–154. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources