The path from nucleolar 90S to cytoplasmic 40S pre-ribosomes - PubMed (original) (raw)
The path from nucleolar 90S to cytoplasmic 40S pre-ribosomes
Thorsten Schäfer et al. EMBO J. 2003.
Abstract
Recent reports have increased our knowledge of the consecutive steps during 60S ribosome biogenesis substantially, but 40S subunit formation is less well understood. Here, we investigate the maturation of nucleolar 90S pre-ribosomes into cytoplasmic 40S pre-ribosomes. During the transition from 90S to 40S particles, the majority of non-ribosomal proteins (approximately 30 species) dissociate, and significantly fewer factors associate with 40S pre-ribosomes. Notably, some of these components are part of both early 90S and intermediate 40S pre-particles in the nucleolus (e.g. Enp1p, Dim1p and Rrp12p), whereas others (e.g. Rio2p and Nob1p) are found mainly on late cytoplasmic pre-40S subunits. Finally, temperature-sensitive mutants mapping either in earlier (enp1-1) or later (rio2-1) components exhibit defects in the formation and nuclear export of pre-40S subunits. Our data provide an initial biochemical map of the pre-40S ribosomal subunit on its path from the nucleolus to the cytoplasm. This pathway involves fewer changes in composition than seen during 60S biogenesis.
Figures
Fig. 1. Enp1p is associated with 90S and 40S pre-ribosomes. (A) The sedimentation behavior of TAP-tagged Enp1p was analyzed by sucrose density gradient centrifugation. A whole-cell lysate (WCL) and fractions 5–32 from the sucrose gradient were analyzed by OD254nm measurement (upper graph) and western blotting using anti-protein A antibodies to reveal the position of TAP-tagged Enp1p (lower panel). A WCL and the pooled 40S and 90S fractions were subjected to tandem affinity purification (TAP). (B) TAP-tagged Enp1p was isolated from an unfractionated yeast WCL, from the 90S pool and the 40S pool by the TAP method. Co-purifying proteins were separated on a 4–12% SDS–polyacrylamide gradient gel, stained with Coomassie Blue and identified by mass spectrometry (see also Table I). Major non-ribosomal proteins of the 40S pre-ribosomes are marked by open circles, high molecular weight factors of the 90S pre-ribosomes by asterisks. Bands 1–29 are 90S factors identified by MS and listed in Table I. Proteins in the low molecular weight range of the gel are predominantly ribosomal Rps proteins (see also Table I).
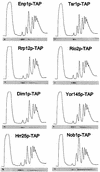
Fig. 2. Sedimentation behavior of TAP-tagged proteins associated with 40S pre-ribosomes on sucrose density gradients. Ribosomal fractions (40S, 60S, 80S and polysomes) were determined by OD254nm measurement of the gradient fractions (upper graph), and the indicated TAP-tagged protein baits were visualized by western blot analysis of these gradient fractions using anti-protein A antibodies (lower panel).
Fig. 3. Subcellular localization of GFP-tagged protein baits associated with 40S pre-ribosomes. The in vivo location of the indicated tagged proteins, associated with different 40S pre-ribosomes, was analyzed in the fluorescence microscope. For microscopic inspection, cells were grown to mid-log phase, mounted on a microscopic slide and photographed with identical exposure times. Strains were also transformed with pRS314-DsRed-NOP1 as a marker for the nucleolus and stained with DAPI to visualize DNA (see Supplementary figure 1).
Fig. 4. Isolation of late cytoplasmic 40S pre-ribosomes. The indicated TAP-tagged protein baits were isolated from yeast lysates by the TAP method, and eluates were separated on a 4–12% SDS–polyacrylamide gradient gel followed by Coomassie Blue staining. Bait proteins are indicated by open circles. Prominent co-purifying proteins were identified by mass spectrometry (see also Table II). Non-ribosomal factors of the pre-40S particels are labeled. As a marker for 90S pre-ribosomes, TAP-purified Noc4p was also loaded on the gel (see also Grandi et al., 2002). High molecular weight 90S factors are marked by asterisks. Putative contaminants in the preparations (indicated by diamonds) are (from top to bottom): Ssa1/2p, Ssb1/2p, Pab1p, eEF-1α, Rpl3p, Rpl4p and Escherichia coli matrix porin f.
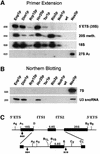
Fig. 5. RNA analysis of the different pre-40S particles. (A) Primer extension to detect 35S, 20Smeth., 18S and 27SA2 rRNA, and (B) northern hybridization to detect 7S rRNA and U3 snoRNA were performed with RNA extracted from affinity-purified TAP-tagged Enp1p, Dim1p, Rrp12p, Hrr25p, Tsr1p, Rio2p, Nob1p, Nsa3p, and a non-tagged wild-type strain (negative control). Oligonucleotides used are indicated to the left of each corresponding panel, and their annealing position in the pre-rRNAs is depicted in (C).
Fig. 6. Rio2p, Yor145p, Tsr1p and Nob1p accumulate in the nucleus of a leptomycin-sensitive xpo1 mutant. In vivo location of Rio2p–GFP, Yor145p–GFP, Tsr1p–GFP and Nob1p–GFP, all chromosomally integrated at the 3′ end of their respective gene locus in the LMB-sensitive xpo1 mutant, after addition of LMB to the culture medium. The fluorescence signals of the GFP fusion proteins were observed in a fluorescence microscope. Note that Rio2p–GFP, Yor145p–GFP and Tsr1p–GFP accumulate in the nucleus already 20 min after addition of LMB, whereas Nob1p–GFP requires 1–2 h.
Fig. 7. enp1 and rio2 ts mutants as well as a GAL1::TSR1 depletion strain are defective in synthesis and nuclear export of 40S subunits. (A) In vivo assay to analyze ribosomal export of 40S and 60S subunits. Ts mutants were shifted for 4 h to 37°C before nuclear accumulation of Rps2p–eGFP (40S subunit reporter) and Rpl25p–eGFP (60S subunit reporter) was determined by fluorescence microscopy. The GAL1::TSR1 depletion stain was shifted from galactose- to glucose-containing medium for 12 h at 30°C. (B) Analysis of ribosomal and polysomal fractions isolated by sucrose density gradient centrifugation from enp1-1 and rio2-1 ts strains, grown at 23°C or shifted for 4 h to 37°C. The UV profiles (OD254nm) of the sucrose gradient are depicted. (C) Growth properties of RIO2 and rio2-1 strains. Serial dilutions were spotted on YPD plates and incubated either for 3 days at 23°C or 4 days at 30, 33 and 37°C.
Fig. 8. Model of 40S and 60S ribosomal biogenesis and export. For description, see text.
Similar articles
- 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm.
Nissan TA, Bassler J, Petfalski E, Tollervey D, Hurt E. Nissan TA, et al. EMBO J. 2002 Oct 15;21(20):5539-47. doi: 10.1093/emboj/cdf547. EMBO J. 2002. PMID: 12374754 Free PMC article. - Nuclear export and cytoplasmic maturation of ribosomal subunits.
Zemp I, Kutay U. Zemp I, et al. FEBS Lett. 2007 Jun 19;581(15):2783-93. doi: 10.1016/j.febslet.2007.05.013. Epub 2007 May 11. FEBS Lett. 2007. PMID: 17509569 Review. - Pre-ribosomes on the road from the nucleolus to the cytoplasm.
Tschochner H, Hurt E. Tschochner H, et al. Trends Cell Biol. 2003 May;13(5):255-63. doi: 10.1016/s0962-8924(03)00054-0. Trends Cell Biol. 2003. PMID: 12742169 Review. - Maturation and intranuclear transport of pre-ribosomes requires Noc proteins.
Milkereit P, Gadal O, Podtelejnikov A, Trumtel S, Gas N, Petfalski E, Tollervey D, Mann M, Hurt E, Tschochner H. Milkereit P, et al. Cell. 2001 May 18;105(4):499-509. doi: 10.1016/s0092-8674(01)00358-0. Cell. 2001. PMID: 11371346 - Late cytoplasmic maturation of the small ribosomal subunit requires RIO proteins in Saccharomyces cerevisiae.
Vanrobays E, Gelugne JP, Gleizes PE, Caizergues-Ferrer M. Vanrobays E, et al. Mol Cell Biol. 2003 Mar;23(6):2083-95. doi: 10.1128/MCB.23.6.2083-2095.2003. Mol Cell Biol. 2003. PMID: 12612080 Free PMC article.
Cited by
- The von Hippel-Lindau protein pVHL inhibits ribosome biogenesis and protein synthesis.
Zhao WT, Zhou CF, Li XB, Zhang YF, Fan L, Pelletier J, Fang J. Zhao WT, et al. J Biol Chem. 2013 Jun 7;288(23):16588-16597. doi: 10.1074/jbc.M113.455121. Epub 2013 Apr 23. J Biol Chem. 2013. PMID: 23612971 Free PMC article. - Targeted proteomics reveals compositional dynamics of 60S pre-ribosomes after nuclear export.
Altvater M, Chang Y, Melnik A, Occhipinti L, Schütz S, Rothenbusch U, Picotti P, Panse VG. Altvater M, et al. Mol Syst Biol. 2012;8:628. doi: 10.1038/msb.2012.63. Mol Syst Biol. 2012. PMID: 23212245 Free PMC article. - The RNA Helicase Ded1 from Yeast Is Associated with the Signal Recognition Particle and Is Regulated by SRP21.
Yeter-Alat H, Belgareh-Touzé N, Le Saux A, Huvelle E, Mokdadi M, Banroques J, Tanner NK. Yeter-Alat H, et al. Molecules. 2024 Jun 20;29(12):2944. doi: 10.3390/molecules29122944. Molecules. 2024. PMID: 38931009 Free PMC article. - Yeast ribosomal protein L40 assembles late into precursor 60 S ribosomes and is required for their cytoplasmic maturation.
Fernández-Pevida A, Rodríguez-Galán O, Díaz-Quintana A, Kressler D, de la Cruz J. Fernández-Pevida A, et al. J Biol Chem. 2012 Nov 2;287(45):38390-407. doi: 10.1074/jbc.M112.400564. Epub 2012 Sep 20. J Biol Chem. 2012. PMID: 22995916 Free PMC article. - Protein phosphorylation and its role in archaeal signal transduction.
Esser D, Hoffmann L, Pham TK, Bräsen C, Qiu W, Wright PC, Albers SV, Siebers B. Esser D, et al. FEMS Microbiol Rev. 2016 Sep;40(5):625-47. doi: 10.1093/femsre/fuw020. Epub 2016 Jul 29. FEMS Microbiol Rev. 2016. PMID: 27476079 Free PMC article. Review.
References
- Angermayr M., Roidl,A. and Bandlow,W. (2002) Yeast Rio1p is the founding member of a novel subfamily of protein serine kinases involved in the control of cell cycle progression. Mol. Microbiol., 44, 309–324. - PubMed
- Baßler J., Grandi,P., Gadal,O., Leßmann,T., Tollervey,D., Lechner,J. and Hurt,E.C. (2001) Identification of a 60S pre-ribosomal particle that is closely linked to nuclear export. Mol. Cell, 8, 517–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases