Prospective identification of tumorigenic breast cancer cells - PubMed (original) (raw)
Prospective identification of tumorigenic breast cancer cells
Muhammad Al-Hajj et al. Proc Natl Acad Sci U S A. 2003.
Erratum in
- Proc Natl Acad Sci U S A. 2003 May 27;100(11):6890
Abstract
Breast cancer is the most common malignancy in United States women, accounting for >40,000 deaths each year. These breast tumors are comprised of phenotypically diverse populations of breast cancer cells. Using a model in which human breast cancer cells were grown in immunocompromised mice, we found that only a minority of breast cancer cells had the ability to form new tumors. We were able to distinguish the tumorigenic (tumor initiating) from the nontumorigenic cancer cells based on cell surface marker expression. We prospectively identified and isolated the tumorigenic cells as CD44(+)CD24(-/low)Lineage(-) in eight of nine patients. As few as 100 cells with this phenotype were able to form tumors in mice, whereas tens of thousands of cells with alternate phenotypes failed to form tumors. The tumorigenic subpopulation could be serially passaged: each time cells within this population generated new tumors containing additional CD44(+)CD24(-/low)Lineage(-) tumorigenic cells as well as the phenotypically diverse mixed populations of nontumorigenic cells present in the initial tumor. The ability to prospectively identify tumorigenic cancer cells will facilitate the elucidation of pathways that regulate their growth and survival. Furthermore, because these cells drive tumor development, strategies designed to target this population may lead to more effective therapies.
Figures
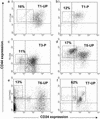
Figure 1
Isolation of tumorigenic cells. Flow cytometry was used to isolate subpopulations of T1 (a and b), T3 (c), T5 (d), T6 (e), and T7 (f) cells that were tested for tumorigenicity in NOD/SCID mice. T1 (b) and T3 (c) had been passaged (P) once in NOD/SCID mice, whereas the rest of the cells were frozen or unfrozen samples obtained directly after removal from a patient (UP). Cells were stained with antibodies against CD44, CD24, Lineage markers, and mouse-H2K (for passaged tumors obtained from mice), and 7AAD. Dead cells (7AAD+), mouse cells (H2K+), and Lineage+ normal cells were eliminated from all analyses. Each plot depicts the CD24 and CD44 staining patterns of live human Lineage− cancer cells, and the frequency of the boxed tumorigenic cancer population as a percentage of cancer cells in each specimen is shown.
Figure 2
DNA content of tumorigenic and nontumorigenic breast cancer cells. The cell cycle status of the ESA+CD44+CD24−/lowLineage− tumorigenic cells (a) and the remaining Lineage− nontumorigenic cancer cells (b) isolated from T1 were determined by Hoechst 33342 staining of DNA content (20). The tumorigenic and nontumorigenic cell populations exhibited similar cell cycle distributions.
Figure 3
Histology from the CD24+ injection site (a; ×20 objective magnification) revealed only normal mouse tissue, whereas the CD24−/low injection site (b; ×40 objective magnification) contained malignant cells. (c) A representative tumor in a mouse at the CD44+CD24−/lowLineage− injection site, but not at the CD44+CD24+Lineage− injection site. T3 cells were stained with Papanicolaou stain and examined microscopically (×100 objective). Both the nontumorigenic (d) and tumorigenic (e) populations contained cells with a neoplastic appearance, with large nuclei and prominent nucleoli.
Figure 4
Phenotypic diversity in tumors arising from CD44+CD24−/lowLineage− cells. The plots depict the CD24 and CD44 or ESA staining patterns of live human Lineage− cancer cells from T1 (a, c, and e) or T2 (b, d, and f). T1 CD44+Lineage− cells (a) or T2 Lineage− cells (b) were obtained from tumors that had been passaged once in NOD/SCID mice. ESA+CD44+CD24−/lowLineage− tumorigenic cells from T1 (c) or CD44+CD24−/lowLineage− tumorigenic cells from T2 (d) were isolated and injected into the breasts of NOD/SCID mice; e and f depict analyses of the tumors that arose from these cells. In both cases, the tumorigenic cells formed tumors that contained phenotypically diverse cells similar to those observed in the original tumor.
Comment in
- Breast cancer stem cells revealed.
Dick JE. Dick JE. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3547-9. doi: 10.1073/pnas.0830967100. Epub 2003 Mar 25. Proc Natl Acad Sci U S A. 2003. PMID: 12657737 Free PMC article. No abstract available. - Breast cancer stem cells: initiating a new sort of thinking.
Herschkowitz JI. Herschkowitz JI. Dis Model Mech. 2010 May-Jun;3(5-6):257-8. doi: 10.1242/dmm.005207. Dis Model Mech. 2010. PMID: 20427552 No abstract available.
Similar articles
- Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells.
Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou J, Burchell JM. Grimshaw MJ, et al. Breast Cancer Res. 2008;10(3):R52. doi: 10.1186/bcr2106. Epub 2008 Jun 9. Breast Cancer Res. 2008. PMID: 18541018 Free PMC article. - Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics.
Calcagno AM, Salcido CD, Gillet JP, Wu CP, Fostel JM, Mumau MD, Gottesman MM, Varticovski L, Ambudkar SV. Calcagno AM, et al. J Natl Cancer Inst. 2010 Nov 3;102(21):1637-52. doi: 10.1093/jnci/djq361. Epub 2010 Oct 8. J Natl Cancer Inst. 2010. PMID: 20935265 Free PMC article. - Identification of pancreatic cancer stem cells.
Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Li C, et al. Cancer Res. 2007 Feb 1;67(3):1030-7. doi: 10.1158/0008-5472.CAN-06-2030. Cancer Res. 2007. PMID: 17283135 - Stem cells in mammary development and carcinogenesis: implications for prevention and treatment.
Dontu G, Liu S, Wicha MS. Dontu G, et al. Stem Cell Rev. 2005;1(3):207-13. doi: 10.1385/SCR:1:3:207. Stem Cell Rev. 2005. PMID: 17142857 Review. - Stem cells: their role in breast cancer development and resistance to treatment.
Nicolini A, Ferrari P, Fini M, Borsari V, Fallahi P, Antonelli A, Berti P, Carpi A, Miccoli P. Nicolini A, et al. Curr Pharm Biotechnol. 2011 Feb 1;12(2):196-205. doi: 10.2174/138920111794295657. Curr Pharm Biotechnol. 2011. PMID: 21044007 Review.
Cited by
- Particle Therapy for Breast Cancer: Benefits and Challenges.
Luo W, Ali YF, Liu C, Wang Y, Liu C, Jin X, Zhou G, Liu NA. Luo W, et al. Front Oncol. 2021 May 5;11:662826. doi: 10.3389/fonc.2021.662826. eCollection 2021. Front Oncol. 2021. PMID: 34026640 Free PMC article. Review. - Metformin: a case of divide and conquer.
Anastasiou D. Anastasiou D. Breast Cancer Res. 2013 Mar 11;15(2):306. doi: 10.1186/bcr3387. Breast Cancer Res. 2013. PMID: 23510106 Free PMC article. - Mini-Review: PDPK1 (3-phosphoinositide dependent protein kinase-1), An Emerging Cancer Stem Cell Target.
Domrachev B, Singh S, Li D, Rudloff U. Domrachev B, et al. J Cancer Treatment Diagn. 2021 Apr 30;5(1):30-35. doi: 10.29245/2578-2967/2021/1.1194. J Cancer Treatment Diagn. 2021. PMID: 34079928 Free PMC article. - Circulating tumor cell enrichment based on physical properties.
Harouaka RA, Nisic M, Zheng SY. Harouaka RA, et al. J Lab Autom. 2013 Dec;18(6):455-68. doi: 10.1177/2211068213494391. Epub 2013 Jul 5. J Lab Autom. 2013. PMID: 23832928 Free PMC article. Review. - Upregulation of circulating cancer stem cell marker, DCLK1 but not Lgr5, in chemoradiotherapy-treated colorectal cancer patients.
Mirzaei A, Tavoosidana G, Modarressi MH, Rad AA, Fazeli MS, Shirkoohi R, Tavakoli-Yaraki M, Madjd Z. Mirzaei A, et al. Tumour Biol. 2015 Jun;36(6):4801-10. doi: 10.1007/s13277-015-3132-9. Epub 2015 Jan 29. Tumour Biol. 2015. PMID: 25631749
References
- Stockler M, Wilcken N R, Ghersi D, Simes R J. Cancer Treat Rev. 2000;26:151–168. - PubMed
- Schultz L B, Weber B L. Curr Opin Oncol. 1999;11:429–434. - PubMed
- Golub T R. N Engl J Med. 2001;344:601–602. - PubMed
- Heppner G H. Cancer Res. 1984;44:2259–2265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous