Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway - PubMed (original) (raw)
Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway
Sung Hee Baek et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Pitx2 is a bicoid-related homeodomain factor that is required for effective cell type-specific proliferation directly activating a specific growth-regulating gene cyclin D2. Here, we report that Pitx2, in response to the Wntbeta-catenin pathway and growth signals, also can regulate c-Myc and cyclin D1. Investigation of molecular mechanisms required for Pitx2-dependent proliferation, in these cases, further supports a nuclear role for beta-catenin in preventing the histone deacetylase 1-dependent inhibitory functions of several DNA-binding transcriptional repressors, potentially including E2F4p130 pocket protein inhibitory complex, as well as lymphoid enhancer factor 1 and Pitx2, by dismissal of histone deacetylase 1 and loss of its enzymatic activity. Thus, beta-catenin plays a signal-integrating role in Wnt- and growth factor-dependent proliferation events in mammalian development by both derepressing several classes of repressors and by activating Pitx2, regulating the activity of several growth control genes.
Figures
Figure 1
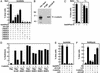
Pitx2 modulates a subset of G1 cell cycle control gene. (A) Recruitment of Pitx2, but not LEF1, to the murine Cyclin D1 promoter in C2C12 cells. A schematic of response elements is shown, with a potential (not proven) LEF1 site. Serum + LiCl caused rapid induction of Cyclin D1 transcripts by RT-PCR in C2C12 cells. A ChIP analysis reveals recruitment of Pitx2, but not LEF1, in response to LiCl treatment, even when we used primers out to −2 kb. (B) Pitx2 and β-catenin on the c-Myc promoter. Murine C2C12 myoblast cells were treated with lithium for 1 h in the presence of serum. ChIP assay was performed by using αPitx2, αHDAC1, and αβ-catenin-specific IgGs. In the absence of lithium, Pitx2 and HDAC1 were bound on the c-Myc promoter in C2C12 cells, whereas, after induction with lithium for 1 h, Pitx2 binding was stronger and release of HDAC1 and the presence of β-catenin were noted. PCR analysis was performed by using oligonucleotide primers flanking the Pitx2- and E2F-binding sites on the murine c-Myc promoter. Schematic shows the location of Pitx2 (−212; −188) and E2F (−154) elements. RT-PCR analysis was performed to check message level after treatment with lithium for 1 h. (C) Role of Pitx2 in c-Myc expression. C2C12 cells were microinjected with αPitx2 IgG and a c-Myc promoter carrying LacZ as a reporter. αPitx2 IgG inhibits the serum-dependent stimulation of the LacZ expression by 70%.
Figure 2
Dismissal of HDAC activity by β-catenin. (A) Coinjection of specific IgGs against HDAC1 or HDAC2, but not HDAC3, HDAC4, HDAC5, or HDAC6, and, to a lesser extent, αN-CoR IgGs relieves repression by Gal4/Pitx2 in a single-cell nuclear microinjection assay in Rat1 cells. (B) N-CoR and β-catenin interactions. Based on isolation of β-catenin in a yeast two-hybrid screen with RDIII of N-CoR, immunoprecipitation experiments were performed in HCT116 colorectal tumor cells, revealing robust interactions, by using specific αβ-catenin and αN-CoR (Upper). Coimmunoprecipitation from 293 cells transfected with HDAC1 and β-catenin and Western blot analysis were performed by using the indicated IgGs. (C) Expression of Gal4/Pitx2-N′ causes repression in MEFs from N-CoR−/− but not N-CoR+/+ littermates. (D) β-Catenin inhibits N-CoR and HDAC1 deacetylase activity. 293 cells were transfected with expression vectors for Flag-tagged N-CoR or Flag-tagged HDACs in the presence or absence of the β-cateninc expression vector. After immunoprecipitation with αFlag-IgG, HDAC activity was assessed as reported previously (17). N-CoR and HDAC levels were equivalent in all immunoprecipitates, as assessed by using αFlag IgG. (E and F) Failure of β-catenin to reverse β-RAR- or Pit-1-dependent repression. Gal4/Pit-1 repression or β-RAR repression on a DR5-dependent lacZ reporter could not be overcome by expression of β-cateninc or administration of lithium. αN-CoR reversed both β-RAR- and Pit-1-dependent repression. All specific cell nuclear microinjection experiments are mean ± SEM with >300 cells microinjected, and experiments were repeated independently a minimum of three times.
Figure 3
Role of β-catenin in derepression. (A) β-Catenin interacts with E2F4. HA-E2F1 and HA-E2F4 expression vectors were cotransfected with β-cateninc-expressing vectors, and immunoprecipitation was performed with control IgG of αβ-catenin IgG. β-Catenin was interacting strongly with E2F4. (B) β-Catenin dismisses HDAC activity associated with E2F4. 293 cells were transfected with β-cateninc expression vectors and immunoprecipitated with preimmune IgG or αE2F4 IgG, and HDAC activity was measured. (C) β-Cateninc also inhibits HDAC activity on p107- and p130-immunoprecipitated material from 293 cells. (D) Effects of αHDAC1 and αN-CoR IgGs on expression of the c-Myc promoter in Rat1 cells. After 48 h under serum-free conditions, cells were microinjected with either αN-CoR or αHDAC1 IgGs, and serum was added 8 h before assay. Each point is mean ± SEM of >300 microinjected cells; similar results were obtained in two additional experiments of similar design by using C2C12 or Rat1 cells. (E) Effects of lithium stimulation on binding of E2F4, p130, and HDAC1 to the c-Myc promoter in C2C12 cells. C2C12 cells were placed in a serum-free medium for 48 h to synchronize cells, and serum, lithium, or both were added for 1–16 h before harvest for ChIP assay. Consistent with serum-dependent regulation of Pitx2 gene expression in C2C12 cells, Pitx2 was not detected at 1 h on the c-Myc promoter in C2C12 cells under the serum-free conditions (Upper) but was detected in serum-treated cells (Lower). Under both conditions, E2F4, p130, and HDAC1, but not β-catenin, were detected. However, 1 h after lithium addition, HDAC1 was no longer detected and β-catenin was present on the promoter.
Figure 4
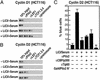
Regulation of cyclin D1 and cyclin D2 by the Wnt pathway in human colon cancer cells. (A and B) ChIP analysis of Cyclin D1 and Cyclin D2 promoters in HCT116 cells, treated as indicated. On the Cyclin D2 promoter, Pitx2 is detected but LEF1 is not detected. On the Cyclin D1 promoter, LEF1 is detected, as is Pitx2. (C) A block of Cyclin D2 expression in HCT116 cells in single-cell nuclear microinjection by αPitx2 IgG or by expression of a dominant-negative form of Pitx2 (Pitx2 N terminus).
Similar articles
- Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development.
Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Kioussi C, et al. Cell. 2002 Nov 27;111(5):673-85. doi: 10.1016/s0092-8674(02)01084-x. Cell. 2002. PMID: 12464179 - The Wnt/beta-catenin-->Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs.
Briata P, Ilengo C, Corte G, Moroni C, Rosenfeld MG, Chen CY, Gherzi R. Briata P, et al. Mol Cell. 2003 Nov;12(5):1201-11. doi: 10.1016/s1097-2765(03)00407-6. Mol Cell. 2003. PMID: 14636578 - Negative regulation of the Wnt-beta-catenin pathway by the transcriptional repressor HBP1.
Sampson EM, Haque ZK, Ku MC, Tevosian SG, Albanese C, Pestell RG, Paulson KE, Yee AS. Sampson EM, et al. EMBO J. 2001 Aug 15;20(16):4500-11. doi: 10.1093/emboj/20.16.4500. EMBO J. 2001. PMID: 11500377 Free PMC article. - Regulation of beta-catenin signaling in the Wnt pathway.
Kikuchi A. Kikuchi A. Biochem Biophys Res Commun. 2000 Feb 16;268(2):243-8. doi: 10.1006/bbrc.1999.1860. Biochem Biophys Res Commun. 2000. PMID: 10679188 Review. - Signaling through beta-catenin and Lef/Tcf.
Novak A, Dedhar S. Novak A, et al. Cell Mol Life Sci. 1999 Oct 30;56(5-6):523-37. doi: 10.1007/s000180050449. Cell Mol Life Sci. 1999. PMID: 11212302 Free PMC article. Review.
Cited by
- Effects of S-Adenosylhomocysteine Hydrolase Downregulation on Wnt Signaling Pathway in SW480 Cells.
Pavičić I, Rokić F, Vugrek O. Pavičić I, et al. Int J Mol Sci. 2023 Nov 8;24(22):16102. doi: 10.3390/ijms242216102. Int J Mol Sci. 2023. PMID: 38003292 Free PMC article. - The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells.
Kulesza A, Paczek L, Burdzinska A. Kulesza A, et al. Biomedicines. 2023 Feb 3;11(2):445. doi: 10.3390/biomedicines11020445. Biomedicines. 2023. PMID: 36830980 Free PMC article. Review. - Inhibitory effect and mechanism of mesenchymal stem cells on liver cancer cells.
Hou L, Wang X, Zhou Y, Ma H, Wang Z, He J, Hu H, Guan W, Ma Y. Hou L, et al. Tumour Biol. 2014 Feb;35(2):1239-50. doi: 10.1007/s13277-013-1165-5. Epub 2013 Oct 18. Tumour Biol. 2014. PMID: 24136741 - Fuz regulates craniofacial development through tissue specific responses to signaling factors.
Zhang Z, Wlodarczyk BJ, Niederreither K, Venugopalan S, Florez S, Finnell RH, Amendt BA. Zhang Z, et al. PLoS One. 2011;6(9):e24608. doi: 10.1371/journal.pone.0024608. Epub 2011 Sep 14. PLoS One. 2011. PMID: 21935430 Free PMC article. - RP58 controls neuron and astrocyte differentiation by downregulating the expression of Id1-4 genes in the developing cortex.
Hirai S, Miwa A, Ohtaka-Maruyama C, Kasai M, Okabe S, Hata Y, Okado H. Hirai S, et al. EMBO J. 2012 Mar 7;31(5):1190-202. doi: 10.1038/emboj.2011.486. Epub 2012 Jan 10. EMBO J. 2012. PMID: 22234186 Free PMC article.
References
- Cadigan K M, Nusse R. Genes Dev. 1997;11:3286–3305. - PubMed
- Ikeya M, Lee S M, Johnson J E, McMahon A P, Takada S. Nature. 1997;389:966–970. - PubMed
- Eastman Q, Grosschedl R. Curr Opin Cell Biol. 1999;11:233–240. - PubMed
- Boutros M, Mlodzik M. Mech Dev. 1999;83:27–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous