Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints - PubMed (original) (raw)
Microarchitecture and protective mechanisms in synovial tissue from clinically and arthroscopically normal knee joints
M D Smith et al. Ann Rheum Dis. 2003 Apr.
Abstract
Background: Synovial biopsies are used to study synovial immunopathology and are increasingly applied for the evaluation of new therapeutic strategies in chronic arthritis. Therefore, it is essential to be informed on the complete spectrum of synovial immunopathology.
Objective: To describe the cellular content, cytokine and cell adhesion molecule expression in synovial tissue from clinically and arthroscopically normal knees.
Methods: Synovial tissue was obtained from 20 normal subjects at the time of knee joint arthroscopy for unexplained knee pain. Tissue sections were studied for basic histopathology and for a range of cell surface markers, cytokines, and cell adhesion molecules by immunoperoxidase staining. Stained sections were evaluated by semiquantitative scoring and digital image analysis.
Results: Normal synovial tissue is composed predominantly of fibrofatty areolar tissue, with a variable thickness of intimal lining, composed of both CD68 positive macrophages and CD55 positive fibroblast-like synoviocytes. Interleukin 1 receptor antagonist (IL1Ra) was frequently detected in the synovial membrane of normal subjects (mean (SD) integrated optical density (IOD)=3809.6 (3893.9)), but both tumour necrosis factor alpha (TNFalpha) and interleukin 1beta (IL1beta) were rarely detected. In addition, cell adhesion molecules were rarely detected in the normal synovial membrane, with the exception of intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Osteoprotegerin (OPG) expression was abundant on synovial lining macrophages (mean (SD) IOD=5276 (4716) as well as endothelial cells (mean (SD) IOD=557 (226)), but receptor activator of nuclear factor kappa ligand (RANKL) expression was rarely seen.
Conclusions: The normal synovial membrane has a variable architecture, including thickness of the lining and the subintimal cell infiltrate, with little inflammatory cytokine production or expression of cell adhesion molecules. The excess of OPG expression over RANKL and IL1Ra over IL1 may be important for protection against joint damage
Figures
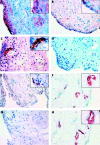
Figure 1
Immunohistochemical labelling of a representative normal synovial membrane stained with (A) anti-CD55 (fibroblast-like synoviocytes), (B) anti-CD68 (macrophages), (C) anti-CD3 (T cells), (D) anti-IL1ß, (E) anti-interleukin 1 receptor antagonist (anti-IL1Ra), (F) anti-factor VIII (endothelial cells), (G) anti-RANKL, and (H) monoclonal antibody 805 for OPG. All images are at x200 magnification except F and H, which are x100 magnification. All inserts in panels A, B, C, and E are x400 magnification, while inserts in panels F and H are x200 magnification.
Similar articles
- Successful treatment of rheumatoid arthritis is associated with a reduction in synovial membrane cytokines and cell adhesion molecule expression.
Smith MD, Slavotinek J, Au V, Weedon H, Parker A, Coleman M, Roberts-Thomson PJ, Ahern MJ. Smith MD, et al. Rheumatology (Oxford). 2001 Sep;40(9):965-77. doi: 10.1093/rheumatology/40.9.965. Rheumatology (Oxford). 2001. PMID: 11561106 Clinical Trial. - Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls.
Haynes DR, Barg E, Crotti TN, Holding C, Weedon H, Atkins GJ, Zannetino A, Ahern MJ, Coleman M, Roberts-Thomson PJ, Kraan M, Tak PP, Smith MD. Haynes DR, et al. Rheumatology (Oxford). 2003 Jan;42(1):123-34. doi: 10.1093/rheumatology/keg047. Rheumatology (Oxford). 2003. PMID: 12509625 - Variability in cytokine and cell adhesion molecule staining in arthroscopic synovial biopsies: quantification using color video image analysis.
Youssef PP, Triantafillou S, Parker A, Coleman M, Roberts-Thomson PJ, Ahern MJ, Smith MD. Youssef PP, et al. J Rheumatol. 1997 Dec;24(12):2291-8. J Rheumatol. 1997. PMID: 9415630 - Macrophages, synovial tissue and rheumatoid arthritis.
Cutolo M, Sulli A, Barone A, Seriolo B, Accardo S. Cutolo M, et al. Clin Exp Rheumatol. 1993 May-Jun;11(3):331-9. Clin Exp Rheumatol. 1993. PMID: 8394794 Review. - Regulation of the expression of adhesion molecules by human synoviocytes.
Lindsley HB, Smith DD, Davis LS, Koch AE, Lipsky PE. Lindsley HB, et al. Semin Arthritis Rheum. 1992 Apr;21(5):330-4. doi: 10.1016/0049-0172(92)90026-a. Semin Arthritis Rheum. 1992. PMID: 1604327 Review.
Cited by
- Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis.
Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M. Bustamante MF, et al. Arthritis Res Ther. 2017 May 31;19(1):110. doi: 10.1186/s13075-017-1303-3. Arthritis Res Ther. 2017. PMID: 28569176 Free PMC article. Review. - Correlation between Focal Nodular Low Signal Changes in Hoffa's Fat Pad Adjacent to Anterior Femoral Cartilage and Focal Cartilage Defect Underlying This Region and Its Possible Implication.
Antony CD, George J, Ng WM, Subramaniam MS. Antony CD, et al. Scientifica (Cairo). 2016;2016:8675160. doi: 10.1155/2016/8675160. Epub 2016 Apr 27. Scientifica (Cairo). 2016. PMID: 27213085 Free PMC article. - Developments in the synovial biology field 2006.
Knedla A, Neumann E, Müller-Ladner U. Knedla A, et al. Arthritis Res Ther. 2007;9(2):209. doi: 10.1186/ar2140. Arthritis Res Ther. 2007. PMID: 17442097 Free PMC article. Review. - Additive effects of inhibiting both mTOR and glutamine metabolism on the arthritis in SKG mice.
Ueda Y, Saegusa J, Okano T, Sendo S, Yamada H, Nishimura K, Morinobu A. Ueda Y, et al. Sci Rep. 2019 Apr 23;9(1):6374. doi: 10.1038/s41598-019-42932-1. Sci Rep. 2019. PMID: 31011190 Free PMC article. - A Pauci-Immune Synovial Pathotype Predicts Inadequate Response to TNFα-Blockade in Rheumatoid Arthritis Patients.
Nerviani A, Di Cicco M, Mahto A, Lliso-Ribera G, Rivellese F, Thorborn G, Hands R, Bellan M, Mauro D, Boutet MA, Giorli G, Lewis M, Kelly S, Bombardieri M, Humby F, Pitzalis C. Nerviani A, et al. Front Immunol. 2020 May 5;11:845. doi: 10.3389/fimmu.2020.00845. eCollection 2020. Front Immunol. 2020. PMID: 32431716 Free PMC article.
References
- Rheumatology (Oxford). 2001 Jun;40(6):623-30 - PubMed
- Rheumatology (Oxford). 2001 Apr;40(4):367-74 - PubMed
- J Immunol. 1992 Aug 1;149(3):1054-62 - PubMed
- J Immunol. 1992 Aug 15;149(4):1424-31 - PubMed
- Br J Rheumatol. 1992 Oct;31(10):653-61 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous