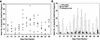Apoptotic cells, including macrophages, are prominent in Theiler's virus-induced inflammatory, demyelinating lesions - PubMed (original) (raw)
Apoptotic cells, including macrophages, are prominent in Theiler's virus-induced inflammatory, demyelinating lesions
Brian P Schlitt et al. J Virol. 2003 Apr.
Abstract
Theiler's murine encephalomyelitis virus (TMEV) persists in the mouse central nervous system principally in macrophages, and infected macrophages in culture undergo apoptosis. We have detected abundant apoptotic cells in perivascular cuffs and inflammatory, demyelinating lesions of SJL mice chronically infected with TMEV. T cells comprised 74% of apoptotic cells, while 8% were macrophages, 0.6% were astrocytes, and approximately 17% remained unidentified. In situ hybridization revealed viral RNA in approximately 1% of apoptotic cells.
Figures
FIG. 1.
(A) Number of apoptotic cells determined by TUNEL assay in spinal cord sections of BeAn virus-infected SJL mice during active demyelinating disease between days 31 and 99. Apoptotic cells in the leptomeninges were excluded from analysis. Shown are mean numbers (○) of TUNEL-positive cells in each spinal cord segment (four to six sections per segment) from mice sacrificed on the indicated days. Bars indicate the mean number of apoptotic cells for each mouse. (B) Number of apoptotic cells (means ± SEs) in different spinal cord locations determined by TUNEL assay in sections of BeAn virus-infected SJL mice during active demyelinating disease between days 31 and 99 (same mice as in panel A).
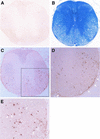
FIG. 2.
Coronal spinal cord sections from a control mouse (A) and a BeAn virus-infected mouse sacrificed on day 69 (B to E). (B) Luxol fast blue staining reveals loss of myelin in the lateral and anterior columns; intense inflammatory infiltrates are visible in the leptomeninges in the ventral root entry zone and anterior commissure even at low-power magnification (×50). (C) Replicate section of panel B stained for TUNEL (no counterstaining), showing TUNEL-positive cells distributed in the white matter, especially anteriolaterally (magnification, ×50). (D) Magnification of the area boxed in panel C, showing distribution of TUNEL-positive cells around the periphery of a demyelinated area (compare with panel B). Arrows indicate TUNEL-positive cells in the pia layers of the leptomeninges (magnification, ×200). (E) Higher magnification (×400) of panel D, showing some TUNEL-positive cells exhibiting characteristic nuclear condensation indicative of apoptosis (arrows).
FIG. 3.
Two activated caspase-3 (blue)- and TUNEL (brown)-stained cord sections from a BeAn virus-infected SJL mouse. Right panel, approximately 20 caspase-3-positive cells; left panel, about 25 caspase-3-positive cells in the white matter and leptomeninges; asterisks in both panels indicate several more prominently TUNEL-stained cells in the white matter (magnification, ×400). No caspase-3 staining was detected in sections from control mice (data not shown).
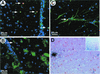
FIG. 4.
Confocal images viewed with an Olympus IMT-2 microscope with digital deconvolution software of frozen spinal cord sections stained for nicked DNA (TUNEL, red); intact nuclei (4′,6′-diamidino-2-phenylindole, blue); and MOMA-2 (A), CD3+ (B), and GFAP (C) fluorescein isothiocyanate-labeled antibodies (green) and light microscopy image of a representative paraffin section taken from a BeAn virus-infected spinal cord on day 63 analyzed by combined in situ hybridization for BeAn virus RNA and TUNEL staining (arrows) for apoptosis (D). Colocalized nuclear and nicked DNA in cells resulted in a yellow-orange color (one cell in panels A and C and four cells in panel B). For combined in situ hybridization for BeAn virus RNA and TUNEL staining (arrows) for apoptosis, antisense and sense 35S-UTP-labeled BeAn virus probes (nucleotides 5967 to 6287) were synthesized with T7 and SP6 polymerase; probes with a specific activity of 6 × 107 cpm/μg in hybridization buffer were applied to tissue sections (D). In panel D, several cells (clustered together) on the extreme left have high viral RNA copy numbers (heavily grained area) and two separate cells on the lower right have low viral RNA copy numbers (asterisks). Only one infected, apoptotic cell, which is the bottommost cell in the cluster on the extreme left, is TUNEL positive (arrow; magnification, ×400). The insert shows the entire coronal spinal cord section from which the higher-power magnification field is taken; note grains over many cells in the anterior and lateral columns indicating the high level of viral persistence in this section (magnification, ×50).
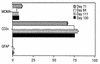
FIG. 5.
Frequency of double-stained (antibodies to MOMA-2, CD3, or GFAP and to TUNEL) apoptotic cells in frozen spinal cord sections of four BeAn virus-infected SJL mice during persistent infection. The mean number (± SE) of double-positive cells identified as macrophages, T lymphocytes, and astrocytes per mouse is shown. In some instances, error bars were below graphic resolution.
Similar articles
- Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler's murine encephalomyelitis virus infection leading to demyelination.
Zheng L, Calenoff MA, Dal Canto MC. Zheng L, et al. J Neuroimmunol. 2001 Aug 30;118(2):256-67. doi: 10.1016/s0165-5728(01)00338-1. J Neuroimmunol. 2001. PMID: 11498260 - [Persistentent infection and demyelination induced by Theiler's murine encephalomyelitis virus (TMEV)].
Ohara Y. Ohara Y. Uirusu. 1999 Dec;49(2):175-81. doi: 10.2222/jsv.49.175. Uirusu. 1999. PMID: 10737115 Review. Japanese. No abstract available. - Infectious RNA isolated from the spinal cords of mice chronically infected with Theiler's murine encephalomyelitis virus.
Libbey JE, Tsunoda I, Whitton JL, Fujinami RS. Libbey JE, et al. J Virol. 2007 Mar;81(6):3009-11. doi: 10.1128/JVI.01663-06. Epub 2006 Sep 27. J Virol. 2007. PMID: 17005653 Free PMC article. - Effects of Keratinocyte-Derived Cytokine (CXCL-1) on the Development of Theiler's Virus-Induced Demyelinating Disease.
Kang MH, Jin YH, Kim BS. Kang MH, et al. Front Cell Infect Microbiol. 2018 Jan 23;8:9. doi: 10.3389/fcimb.2018.00009. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29410948 Free PMC article. - Neuropathogenesis of Theiler's murine encephalomyelitis virus infection, an animal model for multiple sclerosis.
Tsunoda I, Fujinami RS. Tsunoda I, et al. J Neuroimmune Pharmacol. 2010 Sep;5(3):355-69. doi: 10.1007/s11481-009-9179-x. Epub 2009 Nov 6. J Neuroimmune Pharmacol. 2010. PMID: 19894121 Free PMC article. Review.
Cited by
- Activation of tumor suppressor protein p53 is required for Theiler's murine encephalomyelitis virus-induced apoptosis in M1-D macrophages.
Son KN, Pugazhenthi S, Lipton HL. Son KN, et al. J Virol. 2009 Oct;83(20):10770-7. doi: 10.1128/JVI.01030-09. Epub 2009 Aug 5. J Virol. 2009. PMID: 19656889 Free PMC article. - Up-regulation of Fas ligand (FasL) in the central nervous system: a mechanism of immune evasion by rabies virus.
Baloul L, Camelo S, Lafon M. Baloul L, et al. J Neurovirol. 2004 Dec;10(6):372-82. doi: 10.1080/13550280490521122. J Neurovirol. 2004. PMID: 15765808 - Facets of Theiler's Murine Encephalomyelitis Virus-Induced Diseases: An Update.
Gerhauser I, Hansmann F, Ciurkiewicz M, Löscher W, Beineke A. Gerhauser I, et al. Int J Mol Sci. 2019 Jan 21;20(2):448. doi: 10.3390/ijms20020448. Int J Mol Sci. 2019. PMID: 30669615 Free PMC article. Review. - Infectious causes of multiple sclerosis.
Gilden DH. Gilden DH. Lancet Neurol. 2005 Mar;4(3):195-202. doi: 10.1016/S1474-4422(05)01017-3. Lancet Neurol. 2005. PMID: 15721830 Free PMC article. Review. - Theiler's murine encephalomyelitis virus leader protein is the only nonstructural protein tested that induces apoptosis when transfected into mammalian cells.
Fan J, Son KN, Arslan SY, Liang Z, Lipton HL. Fan J, et al. J Virol. 2009 Jul;83(13):6546-53. doi: 10.1128/JVI.00353-09. Epub 2009 Apr 29. J Virol. 2009. PMID: 19403676 Free PMC article.
References
- Bitko, V., and S. Barik. 2001. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell. Biochem. 80:441-454. - PubMed
- Blakemore, W. F., C. J. Welsh, P. Tonks, and A. A. Nash. 1988. Observations on demyelinating lesions induced by Theiler's virus in CBA mice. Acta Neuropathol. 76:581-589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources