What is the origin of pancreatic adenocarcinoma? - PubMed (original) (raw)
Review
What is the origin of pancreatic adenocarcinoma?
Parviz M Pour et al. Mol Cancer. 2003.
Abstract
The concept of pancreatic cancer origin is controversial. Acinar, ductal or islet cells have been hypothesized as the cell of origin. The pros and cons of each of these hypotheses are discussed. Based on the world literature and recent observations, pancreatic cells seem to have potential for phenotypical transdifferentiation, i.e ductal-islet, ductal-acinar, acinar-ductal, acinar-islet, islet-acinar and islet-ductal cells. Although the possibility is discussed that cancer may arise from either islet, ductal or acinar cells, the circumstances favoring the islet cells as the tumor cell origin include their greater transdifferentiation potency into both pancreatic and extrapancreatic cells, the presence of a variety of carcinogen-metabolizing enzymes, some of which are present exclusively in islet cells and the growth factor-rich environment of islets.
Figures
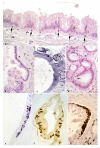
Figure 1
Formation of malignant ductular structures within hamster's islets. a) The lesions is confined to the islets and is sharply demarcated from the surrounding tissue by a layer of fibrosis and inflammatory cells invaded by cancer. H&E × 30. b) Similar lesions showing irregularly shaped malignant glands replacing the islet. H&E × 30. c) In this lesions most part of islet is replaced by malignant gland and sclerosis. Note the sharp delineation of the lesions. Local cancer invasion is seen in the lower portion. H&E × 30. d) An atrophic islet in a patients with chronic pancreatitis. A ductular structure (arrow) is composed of light eosinophilc and clear cells intermingled with islet cells. H&E × 120. e) Another atrophic human islet far remote of a cancer. A dysplastic ductular structure (arrow) in the islet without any signs of depression in the surrounding islet cells. H&E × 120. f) An islet in the vicinity of a well-differentiated adenocarcinoma containing large atypical cells (arrows) intermingled with intact islet cells. H&E × 72. g) A human islet cell in a patient with pancreatic adenocarcinoma loaded with material immunoreactive with anti-MUC-1 antibody (fine granules). Although in these islets the immunoreactivity with anti-insulin has diminished, some granules show reactivity with anti-insulin (arrows). × 104,000, h) Normal human islet. One endocrine cell shows a typical cilia identical to those present in ductal-ductular cells. × 7,200. i) Fine structure of a well-differentiated adenocarcinoma, some cells of which show a few regular or rudimentary granules of endocrine type (arrow). × 7,200.
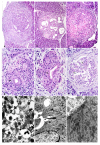
Figure 2
a) Numerous Helle Zelle (light cells) in hyperplastic ductal epithelium corresponding to PanIN2 (arrows). The cells are small with light cytoplasm and hyperchromatic round or oval nuclei. Some cells seem to have two nuclei (the second and third arrows from left). H&E × 120. b) A lesions comparable to PanIN1 to PanIN3 showing clear cells with a small round nuclei surrounded by a halo (arrow). One of the clear cells (arrowhead) presents two nuclei (post mitotic division?). H&E × 120. c) Electron microscopic findings of a similar lesion. Note several clear cells with one or two nuclei. The cells are poor on cell organelles and lay between the basal membran and tumor cells. × 7,200. d) Hyperplastic ductules in an elderly man. Several clear cells are seen within the epithelium. A small group of these cells have interrupted the continuity of the epithelium (bottom arrow) or extended into the stroma (top arrow). H&E × 120. f) Many of the clear cells that can form a circular layer all along the duct, are immunoreactive with antibodies against islet hormones, especially to anti-glucagon and -chromogranin A and against drug-metabolizing CYP450 enzymes. ABC method, Anti-glucagon, × 120. f) A large number of endocrine cells reactive with anti-chromogranin A antibody in a malignant gland. ABC method, × 120. g) A large malignant gland exhibiting papillary infolding of the epithelium. A remarkably large number of overlapping endocrine cells are present in the basal layer of the malignant epithelium. ABC, combination of anti-insulin and anti-glucagon, 120.
Figure 3
a) focal papillary proliferation of the epithelium of the main pancreatic duct of a hamster. Note the lack of continuity between the hyperplastic foci. In such lesions, generally a few or many endocrine cells are present. H&E × 32. b) Part of a malignant epithelium in a male with pancreatic adenocarcinoma. Note the presence of insulin-positive cells (brown in color) at the base of papillary configuration of the epithelium. Mulilabeling with several antibodies against the four islet hormones reveals a larger number of immunoreactive cells. ABC method, × 120.
References
- Schmied BM, Liu G, Matsuzaki H, Ulrich A, Hernberg S, Moyer MP, Weide L, Murphy L, Batra SK, Pour PM. Differentiation of islet cells in long-term culture. Pancreas. 2000;20:337–47. - PubMed
- Schmied BM, Ulrich A, Matsuzaki H, Ding X, Ricordi C, Weide L, Moyer MP, Batra SK, Adrian TE, Pour PM. Transdifferentiation of human islet cells in a long-term culture. Pancreas. 2001;23:157–71. - PubMed
- Schmied BM, Ulrich AB, Matsuzaki H, Ding X, Ricordi C, Moyer MP, Batra SK, Adrian TE, Pour PM. Maintenance of human islets in long term culture. Differentiation. 2000;66:173–80. - PubMed
- Yuan S, Rosenberg L, Paraskevas S, Agapitos D, Duguid WP. Transdifferentiation of human islets to pancreatic ductal cells in collagen matrix culture. Differentiation. 1996;61:67–75. - PubMed
- Kerr-Conte J, Pattou F, Lecomte-Houcke M, Xia Y, Boilly , Proye C, Lefebvre J. Ductal cyst formation in collagen-embedded adult B human islet preparations. A means to the reproduction of nesidioblastosis in vitro. Diabetes. 1996;45:1108–14. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous