Structural requirements of SLP-76 in signaling via the high-affinity immunoglobulin E receptor (Fc epsilon RI) in mast cells - PubMed (original) (raw)
Structural requirements of SLP-76 in signaling via the high-affinity immunoglobulin E receptor (Fc epsilon RI) in mast cells
Alexander Kettner et al. Mol Cell Biol. 2003 Apr.
Abstract
The adapter SLP-76 plays an essential role in Fc epsilon RI signaling, since SLP-76(-/-) bone marrow-derived mast cells (BMMC) fail to degranulate and release interleukin-6 (IL-6) following Fc epsilon RI ligation. To define the role of SLP-76 domains and motifs in Fc epsilon RI signaling, SLP-76(-/-) BMMC were retrovirally transduced with SLP-76 and SLP-76 mutants. The SLP-76 N-terminal and Gads binding domains, but not the SH2 domain, were critical for Fc epsilon RI-mediated degranulation and IL-6 secretion, whereas all three domains are essential for T-cell proliferation following T-cell receptor (TCR) ligation. Unexpectedly, the three tyrosine residues in SLP-76 critical for TCR signaling, Y112, Y128, and Y145, were not essential for IL-6 secretion, but were required for degranulation and mitogen-activated protein kinase activation. Furthermore, a Y112/128F SLP-76 mutant, but not a Y145F mutant, strongly reconstituted mast cell degranulation, suggesting a critical role for Y145 in Fc epsilon RI-mediated exocytosis. These results point to important differences in the function of SLP-76 between T cells and mast cells.
Figures
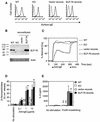
FIG. 1.
Retroviral transduction with SLP-76 reconstitutes FcɛRI-mediated signaling in SLP-76−/− mast cells. (A) IgE receptor expression on BMMC from WT, SLP-76−/− (KO) mice, and SLP-76−/− BMMC reconstituted (reconst.) with control vector or WT SLP-76. Cells were treated with mouse IgE and then incubated with biotinylated anti-IgE and streptavidin-CyChrome (solid line). Control staining was with biotinylated anti-IgE and streptavidin-CyChrome alone (dashed line). (B) SLP-76 protein expression was assessed by immunoblotting after separation of proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (C) Calcium mobilization was detected in BMMC loaded with indo 1-AM. BMMC (10 × 106/ml) were sensitized with IgE (5 μg/ml) for 1 h at room temperature and then stimulated with 25 μg of F(ab′)2 anti-rat Ig per ml followed later by ionomycin (10 μM) at the indicated time points. Results are expressed as percentage of ionomycin-induced calcium mobilization. (D) Release of β-hexosaminidase. BMMC (106) were incubated with 2.5 μg of IgE per ml for 1 h on ice and then stimulated with F(ab′)2 anti-rat Ig at the indicated concentrations. The results represent the mean ± standard deviation of three experiments, each performed in duplicate. **, P < 0.01; *, P < 0.05. ns, not significant as determined by Student's t test compared to WT. (E) IL-6 release by BMMC. BMMC (106) preloaded for 1 h on ice with 2.5 μg of rat IgE per ml were incubated with 1 μg of F(ab′)2 anti-rat Ig per ml for 24 h. IL-6 release was determined by ELISA. The results represent the mean ± standard deviation of four experiments. **, P < 0.01. ns, not significant compared to WT, as determined by Student's t test.
FIG. 2.
Reconstitution of SLP-76−/− BMMC with SLP-76 and SLP-76 mutants. (A) Schematic representation of SLP-76 mutants. (B) IgE receptor expression on BMMC reconstituted with SLP-76 constructs. Cells were labeled as described in the legend to Fig. 1. Levels of FcɛRI expression on WT, KO, and control vector- and WT SLP-76-transduced cells, are shown in Fig. 1A. (C) SLP-76 protein expression in WT, KO, and transduced BMMC as assessed by immunoblotting (Western blotting [WB]) with rabbit anti-SLP-76 antiserum. The membrane was reprobed with anti-PLC-γ2 to control for loading.
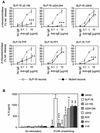
FIG. 3.
IgE-mediated in vitro degranulation and IL-6 release of BMMC reconstituted (reconst.) with SLP-76 constructs. (A) β-Hexosaminidase release of BMMC with mutant construct is shown in comparison with BMMC transduced with WT SLP-76. The results represent the mean ± standard deviation of three experiments. Cells were sensitized and stimulated as described in the legend to Fig. 1C. *, P < 0.05; **, P < 0.01; ***, P < 0.001, as determined by analysis of variance. (B) BMMC were sensitized and stimulated as described in the legend to Fig. 1E, and secreted IL-6 was quantitated 24 h later. The results shown represent the mean ± standard deviation of four experiments. **, P < 0.01; ***, P < 0.001. ns, not significant as determined by Student's t test compared to SLP-76.
FIG. 4.
Calcium mobilization in reconstituted BMMC in response to FcɛRI ligation. Change of fluorescence of the calcium-sensitive dye indo 1-AM was monitored for the indicated time. IgE-sensitized cells were stimulated with F(ab′)2 anti-rat Ig and ionomycin at the indicated time points (▴). BMMC reconstituted (reconst.) with a mutant construct are shown in comparison with SLP-76−/− (KO) or WT BMMC analyzed in parallel. Results are expressed as percentage of ionomycin-induced calcium mobilization. Similar results were obtained in at least five experiments for each of the mutants.
FIG. 5.
Tyrosine phosphorylation of PLC-γ in response to FcɛRI ligation. (A) Tyrosine phosphorylation of SLP-76 and coimmunoprecipitation with btk and Vav. BMMC were sensitized with rat IgE (2.5 μg of rat IgE per ml) followed by cross-linking with F(ab′)2 anti-rat Ig (10 μg/ml) and incubated for 2 min at 37°C. Cell lysates were immunoprecipitated (IP) with rabbit anti-SLP-76 antiserum. Membrane was successively probed with anti-pTyr, anti-btk, anti-SLP-76, and anti-Vav antibodies. The degree of association of SLP-76 with btk and Vav after stimulation was normalized to the signal for SLP-76 as determined by densitometry. Similar results were obtained in two experiments. (B) Tyrosine phosphorylation of PLC-γ1 and PLC-γ2 in WT and SLP-76−/− BMMC. BMMC were sensitized with IgE and stimulated for 7 min as described above. Cell lysates were immunoprecipitated with anti-PLC-γ1 and anti-PLC-γ2. PLC-γ1/2 tyrosine phosphorylation was analyzed by immunoblotting with antiphosphotyrosine antibody (4G10). The membranes were reprobed with anti-PLC-γ1 and anti-PLC-γ2 to control for loading. (C) Tyrosine phosphorylation of PLC-γ2 in SLP-76-reconstituted BMMC. The top two sets of lanes represent a single experiment with cells stimulated simultaneously and then processed in parallel. The bottom set of lanes represents a separate experiment for SLP-76 YYF. Membranes were reprobed with anti-PLC-γ2 to control for loading. Similar results were found in three different experiments. Fold induction normalized to signal for loading was determined by densitometry.
FIG. 6.
Activation of MAPKs in response to FcɛRI ligation in BMMC. BMMC were stimulated for the indicated times and lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Aliquots of the lysates were analyzed in parallel for phosphorylation of ERK1/2 and p38 (A) and SAPK/JNK (B) by Western blotting with the corresponding phosphospecific antibodies. The two sets of lanes in panels A and B represent the same experiment with cells stimulated simultaneously and then processed in parallel. Membranes were reprobed with kinase-specific antibodies to control for loading. Fold induction normalized to signal for loading was determined by densitometry. n.d., not determined.
Similar articles
- Phosphorylation of Tyr342 in the linker region of Syk is critical for Fc epsilon RI signaling in mast cells.
Zhang J, Berenstein E, Siraganian RP. Zhang J, et al. Mol Cell Biol. 2002 Dec;22(23):8144-54. doi: 10.1128/MCB.22.23.8144-8154.2002. Mol Cell Biol. 2002. PMID: 12417718 Free PMC article. - The phospholipase C gamma 1-dependent pathway of Fc epsilon RI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase.
Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, Gilfillan AM. Tkaczyk C, et al. J Biol Chem. 2003 Nov 28;278(48):48474-84. doi: 10.1074/jbc.M301350200. Epub 2003 Sep 16. J Biol Chem. 2003. PMID: 13129935 - Downstream of kinase, p62(dok), is a mediator of Fc gamma IIB inhibition of Fc epsilon RI signaling.
Ott VL, Tamir I, Niki M, Pandolfi PP, Cambier JC. Ott VL, et al. J Immunol. 2002 May 1;168(9):4430-9. doi: 10.4049/jimmunol.168.9.4430. J Immunol. 2002. PMID: 11970986 - Proximal signaling events in Fc epsilon RI-mediated mast cell activation.
Kambayashi T, Koretzky GA. Kambayashi T, et al. J Allergy Clin Immunol. 2007 Mar;119(3):544-52; quiz 553-4. doi: 10.1016/j.jaci.2007.01.017. J Allergy Clin Immunol. 2007. PMID: 17336609 Review. - Fc(epsilon)Ri-dependent signaling pathways in human mast cells.
Tkaczyk C, Gilfillan AM. Tkaczyk C, et al. Clin Immunol. 2001 May;99(2):198-210. doi: 10.1006/clim.2001.4992. Clin Immunol. 2001. PMID: 11318592 Review. No abstract available.
Cited by
- Adapters in the organization of mast cell signaling.
Alvarez-Errico D, Lessmann E, Rivera J. Alvarez-Errico D, et al. Immunol Rev. 2009 Nov;232(1):195-217. doi: 10.1111/j.1600-065X.2009.00834.x. Immunol Rev. 2009. PMID: 19909365 Free PMC article. Review. - Crucial role of SLP-76 and ADAP for neutrophil recruitment in mouse kidney ischemia-reperfusion injury.
Block H, Herter JM, Rossaint J, Stadtmann A, Kliche S, Lowell CA, Zarbock A. Block H, et al. J Exp Med. 2012 Feb 13;209(2):407-21. doi: 10.1084/jem.20111493. Epub 2012 Jan 30. J Exp Med. 2012. PMID: 22291096 Free PMC article. - Anti-microbial cetylpyridinium chloride suppresses mast cell function by targeting tyrosine phosphorylation of Syk kinase.
Obeng B, Bennett LJ, West BE, Wagner DJ, Fleming PJ, Tasker MN, Lorenger MK, Smith DR, Systuk T, Plummer SM, Eom J, Paine MD, Frangos CT, Wilczek MP, Shim JK, Maginnis MS, Gosse JA. Obeng B, et al. J Immunotoxicol. 2024 Dec;21(1):2443397. doi: 10.1080/1547691X.2024.2443397. Epub 2025 Jan 15. J Immunotoxicol. 2024. PMID: 39815634 - The Role of SH2 Domain-containing Leukocyte Phosphoprotein of 76 kDa in the Regulation of Immune Cell Development and Function.
Koretzky GA. Koretzky GA. Immune Netw. 2009 Jun;9(3):75-83. doi: 10.4110/in.2009.9.3.75. Epub 2009 Jun 30. Immune Netw. 2009. PMID: 20107536 Free PMC article. - Signal transduction and chemotaxis in mast cells.
Draber P, Halova I, Polakovicova I, Kawakami T. Draber P, et al. Eur J Pharmacol. 2016 May 5;778:11-23. doi: 10.1016/j.ejphar.2015.02.057. Epub 2015 May 2. Eur J Pharmacol. 2016. PMID: 25941081 Free PMC article. Review.
References
- Bagrodia, S., B. Derijard, R. J. Davis, and R. A. Cerione. 1995. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 270:27995-27998. - PubMed
- Blank, U., C. Ra, L. Miller, K. White, H. Metzger, and J.-P. Kinet. 1989. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature 337:187-189. - PubMed
- Boerth, N. J., J. J. Sadler, D. E. Bauer, J. L. Clements, S. M. Gheith, and G. A. Koretzky. 2000. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J. Exp. Med. 192:1047-1058. - PMC - PubMed
- Bubeck Wardenburg, J., R. Pappu, J. Y. Bu, B. Mayer, J. Chernoff, D. Straus, and A. C. Chan. 1998. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity 9:607-616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases