Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors - PubMed (original) (raw)
Coactivator-dependent acetylation stabilizes members of the SREBP family of transcription factors
Valeria Giandomenico et al. Mol Cell Biol. 2003 Apr.
Abstract
Members of the SREBP family of transcription factors control cholesterol and lipid homeostasis and play important roles during adipocyte differentiation. The transcriptional activity of SREBPs is dependent on the coactivators p300 and CBP. We now present evidence that SREBPs are acetylated by the intrinsic acetyltransferase activity of p300 and CBP. In SREBP1a, the acetylated lysine residue resides in the DNA-binding domain of the protein. Coexpression with p300 dramatically increases the expression of both SREBP1a and SREBP2, and this effect is dependent on the acetyltransferase activity of p300, indicating that acetylation of SREBPs regulates their stability. Indeed, acetylation or mutation of the acetylated lysine residue in SREBP1a stabilizes the protein. We demonstrate that the acetylated residue in SREBP1a is also targeted by ubiquitination and that acetylation inhibits this process. Thus, our studies define acetylation-dependent stabilization of transcription factors as a novel mechanism for coactivators to regulate gene expression.
Figures
FIG. 1.
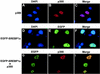
SREBP1a interacts with the coactivator p300. Cos7 cells were transfected with EGFP-SREBP1a and HA-tagged p300, either alone or together, and the subcellular localization of the transfected proteins was determined as described in Materials and Methods. (A to C) p300 (red) is localized to nuclear speckles in cells transfected with p300-HA alone. (D to F) EGFP-SREBP1a (green) shows a uniform nuclear staining in the absence of p300 expression. (G to I) EGFP-SREBP1a (green) is recruited into nuclear speckles when coexpressed with p300-HA (red).
FIG. 2.
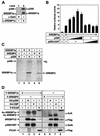
SREBPs are substrates for p300 and CBP. (A) Lysates from HeLa cells grown in lipoprotein-deficient media were immunoprecipitated with anti-Gal4 (lane 1) or anti-SREBP1a (lane 2) antibodies. The immunoprecipitates were washed, and the proteins were separated by SDS-PAGE. Coimmunoprecipitated p300 was detected by Western blotting using anti-p300 antibodies. The amount of mature SREBP1a in the immunoprecipitates was determined with anti-SREBP1a antibodies. (B) HepG2 cells were transfected with SYNSRE-luc in the absence (lane 1) or presence (lanes 2 to 10) of Flag-tagged SREBP1a and in the absence or presence of p300 (25 to 250 ng), either the wild type (lanes 3 to 6) or the ΔHAT1 mutant (lanes 7 to 10). Thirty-six hours after transfection, the luciferase activity was measured. The data represent the averages ± standard deviations of three independent experiments performed in duplicate. (C) His-tagged SREBP1a and SREBP2 were subjected to in vitro acetylation in the absence (lanes 2 and 4) or presence (lanes 3 and 5) of p300 as described in Materials and Methods. After the proteins were separated by SDS-PAGE, the gel was dried and analyzed by phosphorimage analysis. (D) Flag-tagged SREBP1a (lanes 1 to 4) and SREBP2 (lanes 5 to 8) were expressed in 293T cells in the absence or presence of the indicated acetyltransferases. Following immunoprecipitation with anti-Flag antibodies, the samples were resolved by SDS-PAGE and the acetylation of the SREBPs was determined with anti-acetyl lysine antibodies (α-AcK). The levels of SREBPs in the immunoprecipitates were determined with anti-Flag antibodies (α-Flag). The levels of Gal4-p300, Gal4-CBP, and Flag-P/CAF in the cell lysates were determined by Western blotting.
FIG. 3.
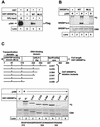
The p300/CBP-binding motif in SREBP1a is important for its acetylation. (A) Flag-tagged p300 was expressed in 293T cells, and the cell lysates were incubated with GST alone (lane 2) or GST-SREBP1a, the wild type (WT; lane 3) and the MLQ mutant (lane 4), coupled to glutathione beads. The complexes were washed, and the proteins were separated by SDS-PAGE. The presence of p300 was detected by Western blotting with anti-Flag antibodies. Ten percent of the material used in the pull-down was loaded on the gel (lane 1). (B) Wild-type GST-SREBP1a and the MLQ mutant were subjected to in vitro acetylation in the absence (lanes 2 and 4) or presence (lanes 3 and 5) of p300 as described in Materials and Methods. After the proteins were separated by SDS-PAGE, the gel was dried and analyzed by phosphorimage analysis. The gel was stained with Coomassie brilliant blue to ensure that equal amounts of GST fusion proteins were used in the assay (CBB). (C) Schematic illustration of the GST-SREBP1a proteins used to map the acetylated residues in SREBP1a. Proteins consisting of GST fused to full-length SREBP1a (amino acids 2 to 490) and the indicated deletion mutants were subjected to in vitro acetylation by using p300 as described in Materials and Methods. After the proteins were separated by SDS-PAGE, the gel was dried and exposed to phosphorimage analysis (top). The gel was stained with Coomassie brilliant blue to ensure that equal amounts of GST fusion proteins were used in the assay (CBB). The core DNA-binding domain (amino acids 312 to 341) of human SREBP1a, including the potential acetylated lysine residues, is shown at the bottom. bHLH-Zip, basic helix-loop-helix zipper.
FIG. 4.
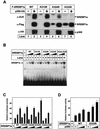
SREBP1a is acetylated in its core DNA-binding domain by p300. (A) Flag-tagged wild-type (WT) SREBP1a and the indicated lysine mutants were expressed in 293T cells in the absence or presence of p300-HA. Following immunoprecipitation of SREBP1a, samples were resolved by SDS-PAGE and the acetylation of SREBP1a was detected with anti-acetyl lysine antibodies (α-AcK). The amount of SREBP1a in the immunoprecipitates was determined with anti-Flag antibodies (α-Flag). The levels of p300-HA in the cell lysates were determined by Western blotting (α-HA). (B) Increasing amounts of in vitro-translated Flag-SREBP1a, the wild type and the indicated mutants, were used in EMSAs with a 32P-labeled probe containing the SRE-1 sequence from the LDLR promoter. (C) Cos7 cells were transfected with SYNSRE-luc in the absence (lane 1) or presence (lanes 2 to 16) of increasing amounts (0.5 to 2.5 ng) of Flag-tagged SREBP1a, the wild type and the indicated mutants. Thirty-six hours after transfection, the luciferase activity was measured. The data represent the averages ± standard deviations (SD) of three independent experiments performed in duplicate. (D) HepG2 cells were transfected with SYNSRE-luc in the absence or presence of Flag-tagged SREBP1a, the wild type and the K333R mutant, in the absence or presence of p300 (25 or 100 ng). Thirty-six hours after transfection, the luciferase activity was measured. The data represent the averages ± SD of three independent experiments performed in duplicate.
FIG. 5.
Acetylation enhances the stability of SREBPs. (A) Flag-tagged wild-type (WT) SREBP1a (lanes 1 to 4) and SREBP2 (lanes 5 to 8) were expressed in 293T cells in the absence or presence of Flag-p300, either the wild type (lanes 2 and 6) or two different acetylation-deficient mutants (lanes 3, 4, 7, and 8). Thirty-six hours after transfection, cell lysates were prepared and resolved by SDS-PAGE. The levels of SREBPs were detected by Western blotting using anti-Flag antibodies (α-Flag). The levels of Flag-p300 in the cell lysates were determined by Western blotting (α-Flag). To ensure that equal amounts of protein were loaded in each well, the levels of Myc-SOCS3 in the samples were estimated by Western blotting using anti-Myc antibodies (α-Myc). (B) Flag-tagged SREBP1a was expressed in 293T cells in the absence (lanes 3 and 4) or presence (lanes 5 and 6) of p300-HA. Thirty-six hours posttransfection, RNA was extracted and resolved on agarose gels, and the mRNA levels for SREBP1a and actin were determined as described in Materials and Methods.
FIG. 6.
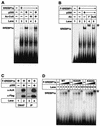
Acetylated SREBP1a binds DNA. (A) Recombinant SREBP1a was used in EMSAs with a 32P-labeled probe containing the SRE-1 sequence from the LDLR promoter. Where indicated, SREBP1a was incubated with p300 (lane 3), acetyl-CoA (Ac-CoA; lane 4), or p300 plus acetyl-CoA (lane 5) prior to the EMSA. (B) Flag-tagged wild-type SREBP1a was expressed in 293T cells in the absence (lane 2) or presence of p300-HA (lanes 3 to 5). Thirty-six hours after transfection, nuclear extracts were prepared and used in EMSA with a 32P-labeled probe containing the SRE-1 sequence from the LDLR promoter. Where indicated, anti-Flag (F; lane 4) or anti-acetyl lysine (AcK; lane 5) antibodies were included in the assay. ∗, supershifted complexes. (C) Nuclear extracts were prepared from 293T cells transfected as in panel B and used in DNAP assays with the SRE-1 sequence from the LDLR promoter (lanes 1 and 2) or immunoprecipitated (IP) with anti-Flag antibodies (lanes 3 and 4). The levels and acetylation of SREBP1a were determined by Western blotting using anti-Flag (α-Flag) and anti-acetyl lysine (α-AcK) antibodies, respectively. (D) Increasing amounts of in vitro-translated Flag-SREBP1a, the wild type (WT) and the indicated mutants, were used in EMSAs with a 32P-labeled probe containing the SRE-1 sequence from the LDLR promoter. The SREBP1a-DNA complexes were supershifted with anti-Flag antibodies (lanes 5, 10, and 15; asterisk).
FIG. 7.
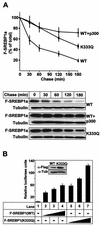
Acetylation stabilizes SREBP1a. (A) 293T cells were transfected with Flag-SREBP1a, either the wild type (WT) or the K333Q mutant, in the absence or presence of p300-HA. Thirty-six hours after transfection, cells were treated with cycloheximide for the indicated times, and the levels of Flag-SREBP1a in the cell lysates were determined as described in Materials and Methods. The amount of SREBP1a at each time point is plotted as a percentage of the amount at the start of the chase and represents the average ± the standard error of the mean of three independent experiments (top). The results from one representative experiment are illustrated at the bottom. To ensure that equal amounts of protein were loaded in each well, the levels of tubulin in the samples were estimated by Western blotting using antitubulin antibodies. (B) HepG2 cells were transfected with SYNSRE-luc in the absence (lane 1) or presence (lanes 2 to 7) of increasing amounts of Flag-SREBP1a (1 to 5 ng), either the wild type (lanes 2 to 4) or K333Q (lanes 5 to 7). Thirty-six hours after transfection, the luciferase activity was measured. The data represent the averages ± standard deviations of three independent experiments per-formed in duplicate. The levels of Flag-SREBP1a in samples 4 (WT) and 7 (K333Q) were determined by Western blotting using anti-Flag antibodies (inset).
FIG. 8.
Acetylation of SREBP1a prevents its ubiquitination. (A) Flag-tagged wild-type SREBP1a and the K333R mutant were expressed in 293T cells in the absence or presence of HA-ubiquitin. Thirty-six hours after transfection, SREBP1a was immunoprecipitated from cell lysates and resolved by SDS-PAGE. The ubiquitination of SREBP1a was determined by Western blotting using anti-HA antibodies (α-HA). The migration of molecular mass standards (in kilodaltons) is indicated to the right. The levels of SREBP1a in the immunoprecipitates were determined by Western blotting using anti-Flag antibodies (α-Flag). (B) GST-SREBP1a was used as the substrate in in vitro ubiquitination reactions in the absence or presence of rabbit reticulocyte lysate (RRL). Where indicated, GST-SREBP1a was acetylated prior to the ubiquitination reaction (lane 3). The samples were resolved by SDS-PAGE, and the ubiquitination of SREBP1a was determined with antiubiquitin antibodies (α-UB). The migration of molecular mass standards (in kilodaltons) is indicated to the right. The amount of SREBP1a and its acetylation were determined with anti-SREBP1a (α-SRE1) and anti-acetyl lysine (α-AcK) antibodies, respectively.
FIG. 9.
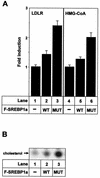
Stabilization of SREBP1a affects cholesterol metabolism. (A) 293T cells were transfected with the vector alone (−) or Flag-tagged wild-type SREBP1a (WT) or K333Q (MUT). Thirty-six hours after transfection, total RNA was extracted and used to determine the expression of the LDLR and HMG-CoA reductase genes by quantitative real-time PCR. The results are presented as the factors by which mRNA levels increased relative to the mRNA levels found in cells transfected with the vector alone. The results represent the averages ± standard deviations of one representative experiment performed in triplicate. (B) 293T cells were transfected with either the vector alone (−) or Flag-tagged wild-type SREBP1a or K333Q (MUT). Thirty-six hours after transfection, cells were placed in fresh media supplemented with [14C]acetate. Nonpolar lipids were extracted and resolved by thin-layer chromatography (TLC). Radioactive products were visualized after exposure of the TLC plates to X-ray film.
Similar articles
- Transcription-dependent degradation controls the stability of the SREBP family of transcription factors.
Sundqvist A, Ericsson J. Sundqvist A, et al. Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):13833-8. doi: 10.1073/pnas.2335135100. Epub 2003 Nov 13. Proc Natl Acad Sci U S A. 2003. PMID: 14615581 Free PMC article. - Acetylation of beta-catenin by p300 regulates beta-catenin-Tcf4 interaction.
Lévy L, Wei Y, Labalette C, Wu Y, Renard CA, Buendia MA, Neuveut C. Lévy L, et al. Mol Cell Biol. 2004 Apr;24(8):3404-14. doi: 10.1128/MCB.24.8.3404-3414.2004. Mol Cell Biol. 2004. PMID: 15060161 Free PMC article. - Acetylation-mediated transcriptional activation of the ETS protein ER81 by p300, P/CAF, and HER2/Neu.
Goel A, Janknecht R. Goel A, et al. Mol Cell Biol. 2003 Sep;23(17):6243-54. doi: 10.1128/MCB.23.17.6243-6254.2003. Mol Cell Biol. 2003. PMID: 12917345 Free PMC article. - Acetylation of the BETA2 transcription factor by p300-associated factor is important in insulin gene expression.
Qiu Y, Guo M, Huang S, Stein R. Qiu Y, et al. J Biol Chem. 2004 Mar 12;279(11):9796-802. doi: 10.1074/jbc.M307577200. Epub 2003 Dec 30. J Biol Chem. 2004. PMID: 14701848 - AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins.
Leff T. Leff T. Biochem Soc Trans. 2003 Feb;31(Pt 1):224-7. doi: 10.1042/bst0310224. Biochem Soc Trans. 2003. PMID: 12546690 Review.
Cited by
- Hepatic glucose sensing is required to preserve β cell glucose competence.
Seyer P, Vallois D, Poitry-Yamate C, Schütz F, Metref S, Tarussio D, Maechler P, Staels B, Lanz B, Grueter R, Decaris J, Turner S, da Costa A, Preitner F, Minehira K, Foretz M, Thorens B. Seyer P, et al. J Clin Invest. 2013 Apr;123(4):1662-76. doi: 10.1172/JCI65538. Epub 2013 Mar 15. J Clin Invest. 2013. PMID: 23549084 Free PMC article. - E4BP4 is an insulin-induced stabilizer of nuclear SREBP-1c and promotes SREBP-1c-mediated lipogenesis.
Tong X, Li P, Zhang D, VanDommelen K, Gupta N, Rui L, Omary MB, Yin L. Tong X, et al. J Lipid Res. 2016 Jul;57(7):1219-30. doi: 10.1194/jlr.M067181. Epub 2016 Jun 1. J Lipid Res. 2016. PMID: 27252523 Free PMC article. - Insulin activates human sterol-regulatory-element-binding protein-1c (SREBP-1c) promoter through SRE motifs.
Dif N, Euthine V, Gonnet E, Laville M, Vidal H, Lefai E. Dif N, et al. Biochem J. 2006 Nov 15;400(1):179-88. doi: 10.1042/BJ20060499. Biochem J. 2006. PMID: 16831124 Free PMC article. - Acetylation of insulin receptor substrate-1 is permissive for tyrosine phosphorylation.
Kaiser C, James SR. Kaiser C, et al. BMC Biol. 2004 Nov 2;2:23. doi: 10.1186/1741-7007-2-23. BMC Biol. 2004. PMID: 15522123 Free PMC article. - Docosahexaenoic acid activates some SREBP-2 targets independent of cholesterol and ER stress in SW620 colon cancer cells.
Størvold GL, Fleten KG, Olsen CG, Follestad T, Krokan HE, Schønberg SA. Størvold GL, et al. Lipids. 2009 Aug;44(8):673-83. doi: 10.1007/s11745-009-3324-4. Epub 2009 Jul 7. Lipids. 2009. PMID: 19582494
References
- Bannister, A. J., E. A. Miska, D. Gorlich, and T. Kouzarides. 2000. Acetylation of importin-alpha nuclear import factors by CBP/p300. Curr. Biol. 10:467-470. - PubMed
- Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8:1243-1254. - PubMed
- Baumann, C. T., H. Ma, R. Wolford, J. C. Reyes, P. Maruvada, C. Lim, P. M. Yen, M. R. Stallcup, and G. L. Hager. 2001. The glucocorticoid receptor interacting protein 1 (GRIP1) localizes in discrete nuclear foci that associate with ND10 bodies and are enriched in components of the 26S proteasome. Mol. Endocrinol. 15:485-500. - PubMed
- Bennett, M. K., and T. F. Osborne. 2000. Nutrient regulation of gene expression by the sterol regulatory element binding proteins: increased recruitment of gene-specific coregulatory factors and selective hyperacetylation of histone H3 in vivo. Proc. Natl. Acad. Sci. USA 97:6340-6344. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous