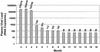Rapid evolution of the neutralizing antibody response to HIV type 1 infection - PubMed (original) (raw)
Rapid evolution of the neutralizing antibody response to HIV type 1 infection
Douglas D Richman et al. Proc Natl Acad Sci U S A. 2003.
Abstract
A recombinant virus assay was used to characterize in detail neutralizing antibody responses directed at circulating autologous HIV in plasma. Examining serial plasma specimens in a matrix format, most patients with primary HIV infection rapidly generated significant neutralizing antibody responses to early (0-39 months) autologous viruses, whereas responses to laboratory and heterologous primary strains were often lower and delayed. Plasma virus continually and rapidly evolved to escape neutralization, indicating that neutralizing antibody exerts a level of selective pressure that has been underappreciated based on earlier, less comprehensive characterizations. These data argue that neutralizing antibody responses account for the extensive variation in the envelope gene that is observed in the early months after primary HIV infection.
Figures
Figure 1
(A) Diagrams of the expression vectors used to generate the pseudovirions used in the neutralization assay. The envelope-defective, luciferase-expressing vector is above the vector that expresses the full-length envelopes amplified from patient plasmas. (B) Schema of the generation of pseudovirions by cotransfection of the two vectors depicted in A. These pseudovirions then are incubated for 1 h with serial 4-fold dilutions of plasma or antibody solutions before infection of the U87-derived target cells to generate luciferase activity.
Figure 2
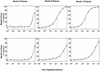
Neutralization of autologous HIV. The neutralizing activity of plasmas obtained from patient 1 at months 0, 6, and 12 after presentation with primary infection is assayed against virus from months 0 and 12. The titer is defined as the reciprocal of the dilution of plasma that produces 50% inhibition of virus replication (dashed lines). The error at each dilution reflects the standard error of duplicate wells.
Figure 3
Plasma HIV viral load in patient TE-1, who initiated potent antiretroviral therapy 16 weeks after presentation (see Table 7). The plasma HIV RNA values over time are shown.
Figure 4
Variable individual autologous neutralizing responses. The autologous neutralizing antibody responses are displayed for seven primary HIV infection patients who declined antiretroviral therapy and for five patients who initiated potent suppressive therapy within 3 months of seroconversion.
Similar articles
- Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals.
Moog C, Fleury HJ, Pellegrin I, Kirn A, Aubertin AM. Moog C, et al. J Virol. 1997 May;71(5):3734-41. doi: 10.1128/JVI.71.5.3734-3741.1997. J Virol. 1997. PMID: 9094648 Free PMC article. - Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses.
Deeks SG, Schweighardt B, Wrin T, Galovich J, Hoh R, Sinclair E, Hunt P, McCune JM, Martin JN, Petropoulos CJ, Hecht FM. Deeks SG, et al. J Virol. 2006 Jun;80(12):6155-64. doi: 10.1128/JVI.00093-06. J Virol. 2006. PMID: 16731954 Free PMC article. - Effect of nonprotective vaccination on antibody response to subsequent human immunodeficiency virus infection.
Pincus SH, Messer KG, Hu SL. Pincus SH, et al. J Clin Invest. 1994 Jan;93(1):140-6. doi: 10.1172/JCI116937. J Clin Invest. 1994. PMID: 8282780 Free PMC article. - Antiviral Therapy by HIV-1 Broadly Neutralizing and Inhibitory Antibodies.
Zhang Z, Li S, Gu Y, Xia N. Zhang Z, et al. Int J Mol Sci. 2016 Nov 18;17(11):1901. doi: 10.3390/ijms17111901. Int J Mol Sci. 2016. PMID: 27869733 Free PMC article. Review. - The role of the humoral immune response in HIV infection.
Fenyö EM, Albert J, McKeating J. Fenyö EM, et al. AIDS. 1996;10 Suppl A:S97-106. doi: 10.1097/00002030-199601001-00014. AIDS. 1996. PMID: 8883616 Review. No abstract available.
Cited by
- Emergence of gp120 V3 variants confers neutralization resistance in an R5 simian-human immunodeficiency virus-infected macaque elite neutralizer that targets the N332 glycan of the human immunodeficiency virus type 1 envelope glycoprotein.
Sadjadpour R, Donau OK, Shingai M, Buckler-White A, Kao S, Strebel K, Nishimura Y, Martin MA. Sadjadpour R, et al. J Virol. 2013 Aug;87(15):8798-804. doi: 10.1128/JVI.00878-13. Epub 2013 May 29. J Virol. 2013. PMID: 23720719 Free PMC article. - Superinfection by discordant subtypes of HIV-1 does not enhance the neutralizing antibody response against autologous virus.
Mayr LM, Powell RL, Ngai JN, Takang WA, Nádas A, Nyambi PN. Mayr LM, et al. PLoS One. 2012;7(6):e38989. doi: 10.1371/journal.pone.0038989. Epub 2012 Jun 14. PLoS One. 2012. PMID: 22720009 Free PMC article. - A Chimeric HIV-1 gp120 Fused with Vaccinia Virus 14K (A27) Protein as an HIV Immunogen.
Vijayan A, García-Arriaza J, Raman SC, Conesa JJ, Chichón FJ, Santiago C, Sorzano CÓ, Carrascosa JL, Esteban M. Vijayan A, et al. PLoS One. 2015 Jul 24;10(7):e0133595. doi: 10.1371/journal.pone.0133595. eCollection 2015. PLoS One. 2015. PMID: 26208356 Free PMC article. - HIV-1 Entry and Membrane Fusion Inhibitors.
Xiao T, Cai Y, Chen B. Xiao T, et al. Viruses. 2021 Apr 23;13(5):735. doi: 10.3390/v13050735. Viruses. 2021. PMID: 33922579 Free PMC article. Review. - Virological features associated with the development of broadly neutralizing antibodies to HIV-1.
Moore PL, Williamson C, Morris L. Moore PL, et al. Trends Microbiol. 2015 Apr;23(4):204-11. doi: 10.1016/j.tim.2014.12.007. Epub 2015 Jan 5. Trends Microbiol. 2015. PMID: 25572881 Free PMC article. Review.
References
- Wyatt R, Kwong P D, Desjardins E, Sweet R W, Robinson J, Hendrickson W A, Sodroski J G. Nature. 1998;393:705–711. - PubMed
- Parren P W, Moore J P, Burton D R, Sattentau Q J. AIDS. 1999;13, Suppl. A:S137–S162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI047745/AI/NIAID NIH HHS/United States
- R37 AI029164/AI/NIAID NIH HHS/United States
- AI 29164/AI/NIAID NIH HHS/United States
- R43-AI 48890/AI/NIAID NIH HHS/United States
- R24 AI106039/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical