Axonal injury heralds virus-induced demyelination - PubMed (original) (raw)
Axonal injury heralds virus-induced demyelination
Ikuo Tsunoda et al. Am J Pathol. 2003 Apr.
Abstract
Axonal pathology has been highlighted as a cause of neurological disability in multiple sclerosis. The Daniels (DA) strain of Theiler's murine encephalomyelitis virus infects the gray matter of the central nervous system of mice during the acute phase and persistently infects the white matter of the spinal cord during the chronic phase, leading to demyelination. This experimental infection has been used as an animal model for multiple sclerosis. The GDVII strain causes an acute fatal polioencephalomyelitis without demyelination. Injured axons were detected in normal appearing white matter at 1 week after infection with DA virus by immunohistochemistry using antibodies specific for neurofilament protein. The number of damaged axons increased throughout time. By 2 and 3 weeks after infection, injured axons were accompanied by parenchymal infiltration of Ricinus communis agglutinin I(+) microglia/macrophages, but never associated with perivascular T-cell infiltration or obvious demyelination until the chronic phase. GDVII virus infection resulted in severe axonal injury in normal appearing white matter at 1 week after infection, without the presence of macrophages, T cells, or viral antigen-positive cells. The distribution of axonal injury observed during the early phase corresponded to regions where subsequent demyelination occurs during the chronic phase. The results suggest that axonal injury might herald or trigger demyelination.
Figures
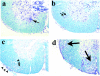
Figure 1.
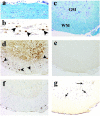
a: One week after DA virus infection, the white matter of the spinal cord appeared normal. In the gray matter, an anterior horn neuron was surrounded by microglia, the process of neuronophagia (arrow; inset, normal neuron and neuronophagia). b: Two weeks after infection the spinal cord appeared primarily normal with a possible increase of cellularity in the NAWM (double arrows; inset, glial star). c: Three weeks after infection meningitis and mild cell infiltration in the parenchyma were evident (arrowhead). Mild vacuolar degeneration of the white matter, possible onset of demyelination, was seen in the VREZ of the white matter (arrowhead). d: One month after infection, significant perivascular infiltration of mononuclear cells and demyelination were detected in the anterior and lateral funiculi of the white matter (large arrow). Luxol fast blue stain. Original magnifications: ×40 (a–d); ×180 (inset in a); ×90 (inset in b).
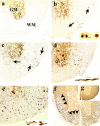
Figure 2.
a–e: Nonphosphorylated NFP immunostain with antibody SMI 311. a: In control mice, nonphosphorylated NFP was detected in neurons and dendrites in the gray matter (GM), but not in the white matter (WM) of the thoracic segment of the transverse section. b: One week after DA virus infection, SMI 311+ axonal swelling (arrows) was detected in the NAWM and SMI 311+ axonal retraction balls were visible in the longitudinal section (inset). c: Two weeks after infection, the number of SMI 311+ axons increased. d: Three weeks after infection, the extent of swelling was severe and coarse varicosities on axons (inset) were seen in the longitudinal section. e: One month after infection, significant axonal swelling occurred in the anterior and lateral funiculi. SMI 311+ fragmented and degenerating axons were shown in the longitudinal section (inset). f and g: Immunostain against phosphorylated NFP showed axonal loss in the white matter (arrowhead) during the chronic phase of DA virus infection (f), but not in the control mice (g). Original magnifications: ×110 (a–e); ×45 (f); ×30 (g); ×300 (insets in b, d, e).
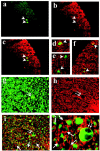
Figure 3.
Double labeling of axons in the white matter of the spinal cord 2 or 3 weeks after DA virus infection with one label detecting MBP+ myelin (rhodamine, red). a–f: Injured axons were detected with antibody against nonphosphorylated NFP (fluorescein isothiocyanate, green). Axonal injury (a) (green, arrowhead) was detected in MBP+ NAWM (b) (red). c–f: Merged images demonstrated injured axons wrapped with MBP+ myelin sheath. g: Phosphorylated NFP labeling (green) visualized not only normal axons but also distended axons (double arrows) that were wrapped with MBP+ myelin sheath (red) (h). i and j: Merged images demonstrated that some myelin sheaths lacked axonal staining (empty myelin, arrow) among intact myelinated axons. Original magnifications: ×230 (a–c); ×100 (d); ×380 (e); ×120 (f); ×230 (g–i); ×710 (j).
Figure 4.
a–d: Time-course study of DA virus persistence. e and f: Double labeling of axons with one label detecting DA virus antigen. a: One week after infection virus antigen was not detected in the spinal cord. b: A small number of virus antigen-positive cells (arrow) were detected in the VREZ, 2 weeks after infection. c: A slight increase of virus antigen-positive cells was noted at 3 weeks after infection. d: DA virus persistence remained at low levels during the chronic phase. e: Usually viral antigen (rhodamine, red, large white arrow) did not co-localize with SMI 311+-damaged axons (fluorescein isothiocyanate, small arrow). f: Viral antigen+ oligodendrocyte (red, rhodamine, center) had processes that wrapped SMI 312+ axons (arrowhead). An empty myelin profile (double arrowhead) was seen in the top center, where viral antigen-positive myelin sheath contained no axon or an SMI 312-negative axon. g–i: In rare instances, viral antigen was seen in axons. In the white matter of the spinal cord, we detected SMI 311 single-positive axons (fluorescein isothiocyanate, green, white arrow), viral antigen single-positive cells (rhodamine, red, large white arrow) and SMI 311/TMEV double-positive axons (yellow, double arrows). Original magnifications: ×110 (a–d); ×230 (e); ×620 (f); ×140 (g–i).
Figure 5.
Kinetics of microglia/macrophage (a–d) and T-cell (e, f) infiltration in the spinal cord in DA virus infection. a: During the acute phase, 1 week after infection, RCA I+ microglia were detected in the gray matter (GM) (arrow), but not in the white matter (WM). In the anterior horn, RCA I+ cells marked the positions of dead neurons (neuronophagia, large arrow, inset). b: Two weeks after infection a cluster of RCA I+ cells (glial star, arrow), which had dendritic processes (inset), was detected in the white matter. c: Three weeks after infection RCA I+ cells developed into rounded phagocytes (inset) and some RCA I+ cells appeared to infiltrate into the parenchyma from the perivascular space and meninges. d: During the chronic phase, a large number of RCA I+ cells were seen in demyelinating lesion of the white matter. e: A consecutive section of (b) showed that CD3+ T cells (arrow) also infiltrated sporadically into the white matter. No perivascular cuffing was detected at this stage. f: Typical perivascular cuffings (arrowhead) and meningitis with CD3+ T cells were seen during the chronic phase, 1 month after infection. a–d: RCA I lectin histochemistry; e and f: anti-CD3 immunohistochemistry. Original magnifications: ×60 (a–d); ×120 (e, f); ×300 (insets in a–c).
Figure 6.
Spinal cord pathology, 1 week after GDVII virus infection (a and b, longitudinal section; c–g, transverse section). a and c: By Luxol fast blue stain, mild meningitis and a few pyknotic neurons in the gray matter (GM) were the only pathological findings; the white matter (WM) appeared normal. b and d: The consecutive sections demonstrated numerous axonal swellings and degeneration positive for nonphosphorylated NFP (arrowhead). Distribution of axonal injury did not correlate with the distribution of viral antigen (e), T cells (f), or microglia/macrophages (g). e: Viral antigen was not detectable by immunohistochemistry in this segment. f: T cells were detected only in the meninges by immunohistochemistry against CD3. g: RCA I+ microglia/macrophages (arrow) were detected in the gray matter. Original magnifications: ×80 (a, b); ×40 (c–g).
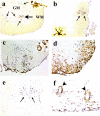
Figure 7.
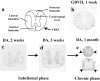
a: Section through a thoracic segment of the mouse spinal cord to demonstrate the subdivisions of the white matter. In transverse section, the spinal cord consists of a butterfly-shaped region of central gray matter and a surrounding mantle of white matter. The white matter of the spinal cord is divided into three funiculi, which are anterior, lateral, and posterior. The corticospinal tract (**) is located in the ventral most portion of the posterior funiculus in rodents. b–d: The distribution of axonal injury was similar for GDVII virus infection at 1 week after infection and DA virus infection at 2 and 3 weeks after infection. Axonal injury in the spinal cord was detected by immunohistochemistry against nonphosphorylated NFP. Thoracic segment images from five mice in each group were superimposed using Image-Pro plus and Adobe Photoshop. e: Luxol fast blue staining showed that demyelination was seen in the anterior and lateral funiculi and the VREZ (arrowhead), but not in the posterior funiculus during the chronic phase of DA virus infection. The distribution of demyelination correlated with regions of axonal injury during the subclinical phase, 2 and 3 weeks after infection. Original magnifications: ×30 (b–d); ×10 (e).
Similar articles
- Neuropathogenesis of Theiler's murine encephalomyelitis virus infection, an animal model for multiple sclerosis.
Tsunoda I, Fujinami RS. Tsunoda I, et al. J Neuroimmune Pharmacol. 2010 Sep;5(3):355-69. doi: 10.1007/s11481-009-9179-x. Epub 2009 Nov 6. J Neuroimmune Pharmacol. 2010. PMID: 19894121 Free PMC article. Review. - Periventricular demyelination and axonal pathology is associated with subependymal virus spread in a murine model for multiple sclerosis.
Kummerfeld M, Seehusen F, Klein S, Ulrich R, Kreutzer R, Gerhauser I, Herder V, Baumgärtner W, Beineke A. Kummerfeld M, et al. Intervirology. 2012;55(6):401-16. doi: 10.1159/000336563. Epub 2012 Apr 25. Intervirology. 2012. PMID: 22538300 - Comparison of Reported Spinal Cord Lesions in Progressive Multiple Sclerosis with Theiler's Murine Encephalomyelitis Virus Induced Demyelinating Disease.
Leitzen E, Jin W, Herder V, Beineke A, Elmarabet SA, Baumgärtner W, Hansmann F. Leitzen E, et al. Int J Mol Sci. 2019 Feb 25;20(4):989. doi: 10.3390/ijms20040989. Int J Mol Sci. 2019. PMID: 30823515 Free PMC article. - Inside-Out versus Outside-In models for virus induced demyelination: axonal damage triggering demyelination.
Tsunoda I, Fujinami RS. Tsunoda I, et al. Springer Semin Immunopathol. 2002;24(2):105-25. doi: 10.1007/s00281-002-0105-z. Springer Semin Immunopathol. 2002. PMID: 12503060 Free PMC article. Review. - Astrocytes, not microglia, are the main cells responsible for viral persistence in Theiler's murine encephalomyelitis virus infection leading to demyelination.
Zheng L, Calenoff MA, Dal Canto MC. Zheng L, et al. J Neuroimmunol. 2001 Aug 30;118(2):256-67. doi: 10.1016/s0165-5728(01)00338-1. J Neuroimmunol. 2001. PMID: 11498260
Cited by
- Autoimmunity-related demyelination in infection by Japanese encephalitis virus.
Tseng YF, Wang CC, Liao SK, Chuang CK, Chen WJ. Tseng YF, et al. J Biomed Sci. 2011 Feb 28;18(1):20. doi: 10.1186/1423-0127-18-20. J Biomed Sci. 2011. PMID: 21356046 Free PMC article. - Transcriptomic meta-analysis of multiple sclerosis and its experimental models.
Raddatz BB, Hansmann F, Spitzbarth I, Kalkuhl A, Deschl U, Baumgärtner W, Ulrich R. Raddatz BB, et al. PLoS One. 2014 Jan 27;9(1):e86643. doi: 10.1371/journal.pone.0086643. eCollection 2014. PLoS One. 2014. PMID: 24475162 Free PMC article. - Protective and detrimental roles for regulatory T cells in a viral model for multiple sclerosis.
Martinez NE, Karlsson F, Sato F, Kawai E, Omura S, Minagar A, Grisham MB, Tsunoda I. Martinez NE, et al. Brain Pathol. 2014 Sep;24(5):436-51. doi: 10.1111/bpa.12119. Epub 2014 Feb 25. Brain Pathol. 2014. PMID: 24417588 Free PMC article. - New aspects of the pathogenesis of canine distemper leukoencephalitis.
Lempp C, Spitzbarth I, Puff C, Cana A, Kegler K, Techangamsuwan S, Baumgärtner W, Seehusen F. Lempp C, et al. Viruses. 2014 Jul 2;6(7):2571-601. doi: 10.3390/v6072571. Viruses. 2014. PMID: 24992230 Free PMC article. Review.
References
- Greenfield JG, King LS: Observations on the histopathology of the cerebral lesions in disseminated sclerosis. Brain 1936, 59:445-458
- Putman TJ: Studies in multiple sclerosis. VII. Similarities between some forms of “encephalomyelitis” and multiple sclerosis. Arch Neurol Psychiatr 1936, 35:1289-1308
- Suzuki K, Andrews JM, Waltz JM, Terry RD: Ultrastructural studies of multiple sclerosis. Lab Invest 1969, 20:444-454 - PubMed
- Ikuta F, Zimmerman HM: Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology 1976, 26:26-28 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources