Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin - PubMed (original) (raw)
Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin
Rania Jounblat et al. Infect Immun. 2003 Apr.
Erratum in
- Infect Immun. 2003 Dec;71(12):7239
Abstract
Pneumolysin, a multifunctional toxin produced by all clinical isolates of Streptococcus pneumoniae, is strongly implicated in the pathogenesis of pneumococcal bronchopneumonia and septicemia. Using isogenic mutant strains, we examined the effect of deletion of the cytotoxic activity or complement-activating activity of pneumolysin on bacterial growth in lungs and blood, histological changes in infected lung tissue, and the pattern of inflammatory cell recruitment. Both of the activities of pneumolysin contributed to the pathology in the lungs, as well as the timing of the onset of bacteremia. Histological changes in the lungs were delayed after infection with either mutant compared to the changes seen after infection with the wild-type pneumococcus. The complement-activating activity of pneumolysin affected the accumulation of T cells, whereas the toxin's cytolytic activity influenced neutrophil recruitment into lung tissue.
Figures
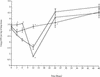
FIG. 1.
Time courses for changes in the numbers of the S. pneumoniae wild-type (□), H+/C− (⋄), and H2−/C+ (○) strains in the lungs of MF1 mice infected intranasally with 106 CFU (n = 10 for each time point). The error bars indicate standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison of the H+/C− or H2−/C+ mutant with the wild-type strain.
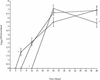
FIG. 2.
Time courses for changes in the numbers of the S. pneumoniae wild-type (□), H+/C− (⋄), and H2−/C+ (○) strains in the blood of MF1 mice infected intranasally with 106 CFU (n = 10 for each time point). The error bars indicate standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison of the H+/C− or H2−/C+ mutant with the wild-type strain.
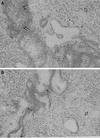
FIG. 3.
Light microscopy of lung tissue from a mouse infected with 106 CFU of S. pneumoniae H+/C− at 24 h postinfection (A), from a mouse infected with 106 CFU of S. pneumoniae H2−/C+ at 24 h postinfection (B), from a mouse infected with 106 CFU of S. pneumoniae H+/C− at 48 h postinfection (C), and from a mouse infected with 106 CFU of S. pneumoniae H2−/C+ at 48 h postinfection (D). In panel A the double arrows indicate heavy cellular infiltrate in infected bronchioles. In panel B the single arrows indicate slight cellular infiltration of infected bronchioles. In panels A and B the open arrows indicate general lung parenchyma that was not involved in inflammation. In panels C and D the thin arrows indicate hypertrophy of the inflamed bronchiole walls, the arrowheads indicate severe multifocal peribronchial infiltration of inflammatory cells, and the thick arrows indicate extensive infiltration of lung parenchyma. Magnification, ×250.
FIG. 3.
Light microscopy of lung tissue from a mouse infected with 106 CFU of S. pneumoniae H+/C− at 24 h postinfection (A), from a mouse infected with 106 CFU of S. pneumoniae H2−/C+ at 24 h postinfection (B), from a mouse infected with 106 CFU of S. pneumoniae H+/C− at 48 h postinfection (C), and from a mouse infected with 106 CFU of S. pneumoniae H2−/C+ at 48 h postinfection (D). In panel A the double arrows indicate heavy cellular infiltrate in infected bronchioles. In panel B the single arrows indicate slight cellular infiltration of infected bronchioles. In panels A and B the open arrows indicate general lung parenchyma that was not involved in inflammation. In panels C and D the thin arrows indicate hypertrophy of the inflamed bronchiole walls, the arrowheads indicate severe multifocal peribronchial infiltration of inflammatory cells, and the thick arrows indicate extensive infiltration of lung parenchyma. Magnification, ×250.
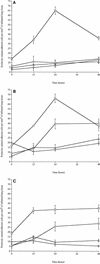
FIG. 4.
Numbers of neutrophils (□), macrophages (⋄), T cells (○), and B cells (▵) in tissue sections from lungs of MF1 mice infected intranasally with 106 CFU of the S. pneumoniae wild-type strain (A), with 106 CFU of S. pneumoniae H+/C− (B), and with 106 CFU of S. pneumoniae H2−/C+ (C) (n = 4 for each time point) The error bars indicate standard errors of the means.
Similar articles
- Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia.
Alexander JE, Berry AM, Paton JC, Rubins JB, Andrew PW, Mitchell TJ. Alexander JE, et al. Microb Pathog. 1998 Mar;24(3):167-74. doi: 10.1006/mpat.1997.0185. Microb Pathog. 1998. PMID: 9514638 - Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia.
Rubins JB, Charboneau D, Paton JC, Mitchell TJ, Andrew PW, Janoff EN. Rubins JB, et al. J Clin Invest. 1995 Jan;95(1):142-50. doi: 10.1172/JCI117631. J Clin Invest. 1995. PMID: 7814608 Free PMC article. - The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus.
Canvin JR, Marvin AP, Sivakumaran M, Paton JC, Boulnois GJ, Andrew PW, Mitchell TJ. Canvin JR, et al. J Infect Dis. 1995 Jul;172(1):119-23. doi: 10.1093/infdis/172.1.119. J Infect Dis. 1995. PMID: 7797901 - Biological properties of pneumolysin.
Mitchell TJ, Andrew PW. Mitchell TJ, et al. Microb Drug Resist. 1997 Spring;3(1):19-26. doi: 10.1089/mdr.1997.3.19. Microb Drug Resist. 1997. PMID: 9109093 Review. - Pneumolysin: a multifunctional pneumococcal virulence factor.
Rubins JB, Janoff EN. Rubins JB, et al. J Lab Clin Med. 1998 Jan;131(1):21-7. doi: 10.1016/s0022-2143(98)90073-7. J Lab Clin Med. 1998. PMID: 9452123 Review.
Cited by
- CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection.
Kadioglu A, Coward W, Colston MJ, Hewitt CR, Andrew PW. Kadioglu A, et al. Infect Immun. 2004 May;72(5):2689-97. doi: 10.1128/IAI.72.5.2689-2697.2004. Infect Immun. 2004. PMID: 15102777 Free PMC article. - Cholesterol as treatment for pneumococcal keratitis: cholesterol-specific inhibition of pneumolysin in the cornea.
Marquart ME, Monds KS, McCormick CC, Dixon SN, Sanders ME, Reed JM, McDaniel LS, Caballero AR, O'Callaghan RJ. Marquart ME, et al. Invest Ophthalmol Vis Sci. 2007 Jun;48(6):2661-6. doi: 10.1167/iovs.07-0017. Invest Ophthalmol Vis Sci. 2007. PMID: 17525197 Free PMC article. - Streptococcus pneumoniae: 'captain of the men of death' and financial burden.
Yesilkaya H, Oggioni MR, Andrew PW. Yesilkaya H, et al. Microbiology (Reading). 2022 Dec;168(12):001275. doi: 10.1099/mic.0.001275. Microbiology (Reading). 2022. PMID: 36748691 Free PMC article. - Pneumolysin Induces 12-Lipoxygenase-Dependent Neutrophil Migration during Streptococcus pneumoniae Infection.
Adams W, Bhowmick R, Bou Ghanem EN, Wade K, Shchepetov M, Weiser JN, McCormick BA, Tweten RK, Leong JM. Adams W, et al. J Immunol. 2020 Jan 1;204(1):101-111. doi: 10.4049/jimmunol.1800748. Epub 2019 Nov 27. J Immunol. 2020. PMID: 31776202 Free PMC article. - PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation.
Pracht D, Elm C, Gerber J, Bergmann S, Rohde M, Seiler M, Kim KS, Jenkinson HF, Nau R, Hammerschmidt S. Pracht D, et al. Infect Immun. 2005 May;73(5):2680-9. doi: 10.1128/IAI.73.5.2680-2689.2005. Infect Immun. 2005. PMID: 15845469 Free PMC article.
References
- Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24:167-174. - PubMed
- Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23:201-209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources