Pulmonary necrosis resulting from DNA vaccination against tuberculosis - PubMed (original) (raw)
Pulmonary necrosis resulting from DNA vaccination against tuberculosis
Jennifer L Taylor et al. Infect Immun. 2003 Apr.
Abstract
The use of DNA constructs encoding mycobacterial proteins is a promising new approach to vaccination against tuberculosis. A DNA vaccine encoding the hsp60 molecule of Mycobacterium leprae has previously been shown to protect against intravenous infection of mice with Mycobacterium tuberculosis in both the prophylactic and immunotherapeutic modes. It is shown here, however, that this vaccine was not effective in a more realistic aerosol infection model or in a model of latent tuberculosis in the lungs. Moreover, when given in an immunotherapeutic model the immunized mice developed classical Koch reactions characterized by multifocal discrete regions of cellular necrosis throughout the lung granulomas. Similar and equally severe reactions were seen in mice given a vaccine with DNA coding for the Ag85 antigen of M. tuberculosis. This previously unanticipated safety problem indicates that DNA vaccines should be used with caution in individuals who may have already been exposed to tuberculosis.
Figures
FIG. 1.
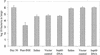
Prophylactic vaccination in C57BL/6 mice. Animals were given DNA or control vector DNA three times and then rested for 1 month prior to low-dose aerosol challenge with M. tuberculosis H37Rv. Controls received injections of saline or a single dose of BCG. Data shown are the mean numbers of bacteria recovered from the lungs 30 days after aerosol exposure (n = 5) (error bars, standard errors of the means). Only mice receiving BCG were protected against the challenge infection (P > 0.01).
FIG. 2.
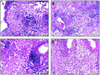
Photomicrographs of lungs of C57BL/6 mice harvested 30 days (A and B) or 80 days (C and D) after aerosol exposure to M. tuberculosis. Mice received prophylactic vaccination with hsp60/lep (A and C) or saline (B and D). Alveoli contain large numbers of macrophages and lower numbers of lymphocytes, but there is no appreciable neutrophil infiltration or necrosis. At day 80 perivascular accumulations of lymphocytes are seen; these were particularly prominent in the hsp60/lep vaccinated animals.
FIG. 3.
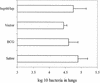
Effect of hsp60/lep vaccine in the modified Cornell model. Four weeks after exposure to low-dose aerosol challenge with M. tuberculosis H37Rv, the bacterial load was assessed (day 30 counts), and then the remaining mice were treated with isoniazid for 60 days, after which the reduction in bacterial load was assessed (Post-INH). Mice were then given three vaccinations with the hsp60/lep DNA or control injections. Thirty days after the final vaccination the bacterial load was determined, and it was then determined again after 8 weeks of treatment with dexamethasone. In each case the mean value is shown (n = 5) (error bars, standard errors of the means).
FIG. 4.
Representative histologic appearance of lungs from BALB/c mice given immunotherapeutic vaccination with hsp60/lep. (A) In mice given saline only small granulomatous foci were seen in the lungs of these animals 130 days after low-dose aerosol challenge with M. tuberculosis H37Rv. Perivascular aggregates of lymphocytes can be seen extending into the adjacent parenchyma, which is variably surrounded by macrophages. Size bar = 100 μm. (B) In mice given hsp60/lep DNA the lesions are very large (size bar = 100 μm) and show evidence of extensive tissue destruction. There is a sharp line of demarcation with normal lung parenchyma, and alveoli are filled with infiltrates of mononuclear cells and some neutrophils. There is loss of alveolar wall detail, with multifocal necrosis. (C) At a higher power of magnification (size bar = 10 μm), these multiple necrotic foci are characterized by fibrillar acidophilic cellular debris with clear clefts that represent extracellular cytoplasmic lipid deposition (arrows). These foci contain a mixture of normal to completely degenerate macrophages. (D) Acid-fast staining revealed multiple bacilli (example indicated with an arrow) visible within the cytoplasm of foamy macrophages as well as in the extracellular space of necrotic foci. Such bacilli were only rarely seen in lesions of control mice at this stage of the infection.
FIG. 5.
Immunotherapeutic vaccination in C57BL/6 mice. Animals were given three injections of DNA, vector DNA control, or BCG on days 60, 81, and 102 after exposure to low-dose aerosol challenge with M. tuberculosis H37Rv. The data shown are the mean numbers of bacteria recovered from the lungs after a further 30 days (n = 5) (error bars, standard errors of the means). No protection was seen in any groups at this time, or after a further seventy days (data not shown).
FIG. 6.
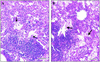
Histologic appearance of lungs of C57BL/6 mice 30 days after the third immunotherapeutic vaccination with hsp60/lep. (A) Alveoli and interalveolar septae are effaced by infiltrates of macrophages, some of which have abundant foamy cytoplasm. There are random foci of necrosis containing karyorhectic and cellular debris with intralesional aggregates of neutrophils (indicated by an arrow). Mice given saline (B) or DNA vector control (C) have prominent perivascular accumulations of lymphocytes and intra-alveolar macrophages but no necrosis or neutrophilic infiltration.
FIG. 7.
Histologic appearance of lungs one hundred days after immunotherapeutic vaccination with hsp60/lep DNA. Foci of necrosis contain debris (small arrows) and clefts of accumulated cholesterol (large arrows). Alveoli contain macrophages with abundant foamy cytoplasm, and aggregates of lymphocytes are prominent. Overall, the appearances of lungs from vaccinated (A) and saline controls (B) were similar.
Similar articles
- Lack of protection in mice and necrotizing bronchointerstitial pneumonia with bronchiolitis in guinea pigs immunized with vaccines directed against the hsp60 molecule of Mycobacterium tuberculosis.
Turner OC, Roberts AD, Frank AA, Phalen SW, McMurray DM, Content J, Denis O, D'Souza S, Tanghe A, Huygen K, Orme IM. Turner OC, et al. Infect Immun. 2000 Jun;68(6):3674-9. doi: 10.1128/IAI.68.6.3674-3679.2000. Infect Immun. 2000. PMID: 10816527 Free PMC article. - The safety of post-exposure vaccination of mice infected with Mycobacterium tuberculosis.
Derrick SC, Perera LP, Dheenadhayalan V, Yang A, Kolibab K, Morris SL. Derrick SC, et al. Vaccine. 2008 Nov 11;26(48):6092-8. doi: 10.1016/j.vaccine.2008.09.011. Epub 2008 Sep 20. Vaccine. 2008. PMID: 18809446 - DNA vaccines against tuberculosis.
Ulmer JB, Montgomery DL, Tang A, Zhu L, Deck RR, DeWitt C, Denis O, Orme I, Content J, Huygen K. Ulmer JB, et al. Novartis Found Symp. 1998;217:239-46; discussion 246-53. doi: 10.1002/0470846526.ch18. Novartis Found Symp. 1998. PMID: 9949812 - [Novel vaccines against M. tuberculosis].
Okada M. Okada M. Kekkaku. 2006 Dec;81(12):745-51. Kekkaku. 2006. PMID: 17240920 Review. Japanese. - Immunotherapy for TB.
Doherty TM. Doherty TM. Immunotherapy. 2012 Jun;4(6):629-47. doi: 10.2217/imt.12.52. Immunotherapy. 2012. PMID: 22788130 Review.
Cited by
- Sequential chemoimmunotherapy of experimental visceral leishmaniasis using a single low dose of liposomal amphotericin B and a novel DNA vaccine candidate.
Seifert K, Juhls C, Salguero FJ, Croft SL. Seifert K, et al. Antimicrob Agents Chemother. 2015 Sep;59(9):5819-23. doi: 10.1128/AAC.00273-15. Epub 2015 Jun 8. Antimicrob Agents Chemother. 2015. PMID: 26055371 Free PMC article. - Mixed-strain mycobacterium tuberculosis infections and the implications for tuberculosis treatment and control.
Cohen T, van Helden PD, Wilson D, Colijn C, McLaughlin MM, Abubakar I, Warren RM. Cohen T, et al. Clin Microbiol Rev. 2012 Oct;25(4):708-19. doi: 10.1128/CMR.00021-12. Clin Microbiol Rev. 2012. PMID: 23034327 Free PMC article. Review. - Permutations of time and place in tuberculosis.
Elkington PT, Friedland JS. Elkington PT, et al. Lancet Infect Dis. 2015 Nov;15(11):1357-60. doi: 10.1016/S1473-3099(15)00135-8. Epub 2015 Aug 28. Lancet Infect Dis. 2015. PMID: 26321650 Free PMC article. Review. - On the use of DNA vaccines for the prophylaxis of mycobacterial diseases.
Huygen K. Huygen K. Infect Immun. 2003 Apr;71(4):1613-21. doi: 10.1128/IAI.71.4.1613-1621.2003. Infect Immun. 2003. PMID: 12654772 Free PMC article. Review. No abstract available. - The tuberculous granuloma: an unsuccessful host defence mechanism providing a safety shelter for the bacteria?
Silva Miranda M, Breiman A, Allain S, Deknuydt F, Altare F. Silva Miranda M, et al. Clin Dev Immunol. 2012;2012:139127. doi: 10.1155/2012/139127. Epub 2012 Jul 3. Clin Dev Immunol. 2012. PMID: 22811737 Free PMC article. Review.
References
- Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. - PubMed
- Dye, C., S. Scheele, P. Dolin, V. Pathania, M. C. Raviglione, et al. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA 282:677-686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous