Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine - PubMed (original) (raw)
Striatal cell type-specific overexpression of DeltaFosB enhances incentive for cocaine
Christina R Colby et al. J Neurosci. 2003.
Abstract
The transcription factor DeltaFosB accumulates in substance P-dynorphin-containing striatal neurons with repeated cocaine use. Here, we show that inducible transgenic DeltaFosB overexpression in this same striatal cell type facilitates acquisition of cocaine self-administration at low-threshold doses, consistent with increased sensitivity to the pharmacological effects of the drug. Importantly, DeltaFosB also enhances the degree of effort mice will exert to maintain self-administration of higher doses on a progressive ratio schedule of reinforcement, whereas levels of cocaine intake are not altered on less demanding fixed-ratio schedules. Acquisition and extinction of behavior reinforced by food pellets is not altered in DeltaFosB-overexpressing mice, indicating that DeltaFosB does not alter the capacity to learn an instrumental response or cause response perseveration in the absence of reinforcement. These data suggest that accumulation of DeltaFosB contributes to drug addiction by increasing the incentive properties of cocaine, an effect that could increase the risk for relapse long after cocaine use ceases.
Figures
Fig. 1.
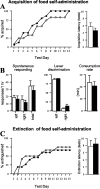
Striatal cell-specific overexpression of ΔFosB (n = 15; filled circles and_bars_) fails to alter acquisition and extinction of lever-press responding reinforced by food pellets compared with bigenic littermate controls maintained on doxycycline (n = 12; open circles and bars).A, The percentage of mice achieving acquisition criteria (for criteria, see Results) in daily 1 hr tests and the mean ± SEM number of test sessions to acquire self-administration of food pellets on an FR3 reinforcement schedule are shown. B, Spontaneous lever-press responding in naive mice before acquisition testing is similar between groups (left), and ΔFosB overexpression also fails to alter lever discrimination on the day acquisition criteria are met (middle) or the latency to consume 30 food pellets after 2 additional training days (right). C, The percentage of ΔFosB mice achieving extinction criteria (≤10 responses in 2 hr at both levers) and the number of test sessions to achieve extinction criteria in the absence of reinforcement are similar in ΔFosB and control mice.
Fig. 2.
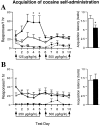
Striatal cell-specific overexpression of ΔFosB (filled circles and bars) facilitates acquisition of cocaine self-administration (FR1) at a low-threshold dose of cocaine (125 μg/kg per injection) (A) but not at a higher suprathreshold dose (250 μg/kg per injection) (B) compared with littermate bigenic controls maintained on doxycycline (open circles and bars). The numbers of cocaine injections (solid lines) and inactive lever presses (dashed lines) are shown at left, and the number of test sessions (latency) to achieve criteria for acquisition of cocaine self-administration are shown at right (for criteria, see Results). Each dose is tested for 5 d, followed by a higher training dose (500 μg/kg per injection) for days 6–10 to demonstrate a capacity for acquisition in all mice used in the analysis. Asterisks indicate that ΔFosB mice (n = 11, 7) differ from littermate controls (n = 13, 10) for threshold–dose cocaine self-administration by tests for simple effects (p < 0.05).
Fig. 3.
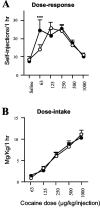
Striatal cell-specific overexpression of ΔFosB increases sensitivity to a low threshold dose of cocaine after acquisition and stabilization of self-administration on an FR5 schedule (n = 16; filled circles) but does not alter self-administration rates at higher doses that are reinforcing in littermate controls (n = 17;open circles) (A) or overall cocaine intake across all doses (B). Black dots indicate that the 63 μg/kg per injection dose differs from saline by Dunnett's test (p < 0.001).
Fig. 4.
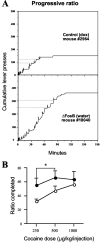
Striatal cell-specific overexpression of ΔFosB facilitates cocaine self-administration on a progressive ratio schedule of reinforcement. A, Cumulative active lever response records for representative mice show that ΔFosB increases the number of cocaine injections (arrows) earned relative to a bigenic littermate control (250 μg/kg per injection). dox, Doxycycline. B, ΔFosB mice (n = 11; filled circles) exert greater effort to maintain cocaine self-administration, as reflected by completing a higher ratio of responses per injection (distance between dotted lines in A) immediately before cessation of self-administration. Convergence of the final ratio completed at the highest injection dose indicates that littermate control mice (n = 17; open circles) are capable of performing at levels found in ΔFosB mice. _Asterisk_indicates main effect of group on ratio completed by ANOVA across the 250 and 500 μg/kg per injection dose (p< 0.05).
Similar articles
- Delta FosB regulates wheel running.
Werme M, Messer C, Olson L, Gilden L, Thorén P, Nestler EJ, Brené S. Werme M, et al. J Neurosci. 2002 Sep 15;22(18):8133-8. doi: 10.1523/JNEUROSCI.22-18-08133.2002. J Neurosci. 2002. PMID: 12223567 Free PMC article. - ΔFosB enhances the rewarding effects of cocaine while reducing the pro-depressive effects of the kappa-opioid receptor agonist U50488.
Muschamp JW, Nemeth CL, Robison AJ, Nestler EJ, Carlezon WA Jr. Muschamp JW, et al. Biol Psychiatry. 2012 Jan 1;71(1):44-50. doi: 10.1016/j.biopsych.2011.08.011. Epub 2011 Sep 29. Biol Psychiatry. 2012. PMID: 21962331 Free PMC article. - Overexpression of DeltaFosB is associated with attenuated cocaine-induced suppression of saccharin intake in mice.
Freet CS, Steffen C, Nestler EJ, Grigson PS. Freet CS, et al. Behav Neurosci. 2009 Apr;123(2):397-407. doi: 10.1037/a0015033. Behav Neurosci. 2009. PMID: 19331462 Free PMC article. - DeltaFosB: a sustained molecular switch for addiction.
Nestler EJ, Barrot M, Self DW. Nestler EJ, et al. Proc Natl Acad Sci U S A. 2001 Sep 25;98(20):11042-6. doi: 10.1073/pnas.191352698. Proc Natl Acad Sci U S A. 2001. PMID: 11572966 Free PMC article. Review. - The neurobiology of cocaine addiction.
Nestler EJ. Nestler EJ. Sci Pract Perspect. 2005 Dec;3(1):4-10. doi: 10.1151/spp05314. Sci Pract Perspect. 2005. PMID: 18552739 Free PMC article. Review.
Cited by
- Preventive role of social interaction for cocaine conditioned place preference: correlation with FosB/DeltaFosB and pCREB expression in rat mesocorticolimbic areas.
El Rawas R, Klement S, Salti A, Fritz M, Dechant G, Saria A, Zernig G. El Rawas R, et al. Front Behav Neurosci. 2012 Mar 2;6:8. doi: 10.3389/fnbeh.2012.00008. eCollection 2012. Front Behav Neurosci. 2012. PMID: 22403532 Free PMC article. - Cocaine self-administration behaviors in ClockΔ19 mice.
Ozburn AR, Larson EB, Self DW, McClung CA. Ozburn AR, et al. Psychopharmacology (Berl). 2012 Sep;223(2):169-77. doi: 10.1007/s00213-012-2704-2. Epub 2012 Apr 26. Psychopharmacology (Berl). 2012. PMID: 22535308 Free PMC article. - Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors.
Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Zhang L, et al. J Neurosci. 2004 Mar 31;24(13):3344-54. doi: 10.1523/JNEUROSCI.0060-04.2004. J Neurosci. 2004. PMID: 15056714 Free PMC article. - Quantitative mapping of cocaine-induced ΔFosB expression in the striatum of male and female rats.
Sato SM, Wissman AM, McCollum AF, Woolley CS. Sato SM, et al. PLoS One. 2011;6(7):e21783. doi: 10.1371/journal.pone.0021783. Epub 2011 Jul 1. PLoS One. 2011. PMID: 21747956 Free PMC article. - Cell-Type-Specific Role of ΔFosB in Nucleus Accumbens In Modulating Intermale Aggression.
Aleyasin H, Flanigan ME, Golden SA, Takahashi A, Menard C, Pfau ML, Multer J, Pina J, McCabe KA, Bhatti N, Hodes GE, Heshmati M, Neve RL, Nestler EJ, Heller EA, Russo SJ. Aleyasin H, et al. J Neurosci. 2018 Jun 27;38(26):5913-5924. doi: 10.1523/JNEUROSCI.0296-18.2018. Epub 2018 Jun 11. J Neurosci. 2018. PMID: 29891732 Free PMC article.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. - PubMed
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. - PubMed
- Aubert I, Ghorayeb I, Normand E, Bloch B. Phenotypical characterization of the neurons expressing the D1 and D2 dopamine receptors in the monkey striatum. J Comp Neurol. 2000;418:22–32. - PubMed
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. - PubMed
- Canales JJ, Graybiel AM. Patterns of gene expression and behavior induced by chronic dopamine treatments. Ann Neurol. 2000;47:S53–S59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DA008227/DA/NIDA NIH HHS/United States
- R29 DA010460/DA/NIDA NIH HHS/United States
- DA-10460/DA/NIDA NIH HHS/United States
- DA-08227/DA/NIDA NIH HHS/United States
- R01 DA010460/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases