Learning-induced arg 3.1/arc mRNA expression in the mouse brain - PubMed (original) (raw)
. 2003 Mar-Apr;10(2):99-107.
doi: 10.1101/lm.53403.
Affiliations
- PMID: 12663748
- PMCID: PMC196661
- DOI: 10.1101/lm.53403
Free PMC article
Learning-induced arg 3.1/arc mRNA expression in the mouse brain
Monique Montag-Sallaz et al. Learn Mem. 2003 Mar-Apr.
Free PMC article
Abstract
The effector immediate-early gene (IEG) arg 3.1, also called arc, encodes a protein interacting with the neuronal cytoskeleton. The selective localization of arg 3.1/arc mRNA in activated dendritic segments suggests that the arg 3.1/arc protein may be synthesized at activated post-synaptic sites and that arg 3.1/arc could participate in structural and functional modifications underlying cognitive processes like memory formation. To analyze whether learning itself is sufficient to trigger expression of arg 3.1/arc, we developed a one-trial learning paradigm in which mice learned to enter a dark compartment to escape from an aversively illuminated area. Arg 3.1/arc mRNA expression was analyzed by in situ hybridization in three groups of mice as follows: a control group with no access to the dark compartment, a learning group having access to the dark compartment for one trial, and a retrieval group having access to the dark compartment for two trials on consecutive days. All animals from the learning and retrieval groups escaped the illuminated area, and those tested 24 h later (retrieval group) showed a strongly reduced latency to enter the dark compartment, demonstrating the validity of our learning paradigm to induce long-term memory. Our results show that acquisition of a simple task results in a brain area-specific biphasic increase in arg 3.1/arc mRNA expression 15 min and 4.5 h post-training. This increase was detected specifically in the learning group but neither in the control nor in the retrieval groups. The pattern of arg 3.1/arc mRNA expression corresponds temporally to the two mRNA- and protein-synthesis-dependent periods of long-term memory formation. Our study provides the first unequivocal evidence that arg 3.1/arc expression is induced by a learning task and strongly suggests a role of arg 3.1/arc mRNA in the early and late cellular mechanisms underlying the stabilization of the memory trace.
Figures
Figure 1
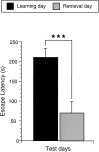
Performance of the learning and retrieval group animals in the light/dark box learning paradigm. Bar graphs illustrating the average latency of the animals of the learning and retrieval groups to enter the dark compartment. The latencies were strongly reduced from the first to the second test day. (Learning day) A total of 19 animals were analyzed; (retrieval day) 10 animals were analyzed. (***)P < 0.001.
Figure 2
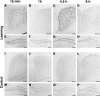
arg 3.1/arc mRNA expression in the cingulate cortex and dentate gyrus detected by non-radioactive in situ hybridization. Frontal sections showing arg 3.1/arc mRNA expression detected by in situ hybridization using a digoxigenin-labeled antisense probe in the cingulate cortex (A–D,I–L) and the dentate gyrus (E–H,M–P) of mice from the learning (A–H) or control (I–P) groups sacrificed 15 min (A,E,I,M), 1 h (B,F,J,N), 4.5 h (C,G,K,O), and 6 h (D,H,L,P) after the test. Two peaks of arg 3.1 expression in the cingulate cortex are clearly visible for the animals of the learning group at the 15-min and 4.5-h time points. In the dentate gyrus, some strongly labeled cells are always visible, and were used as control for the reaction. Scale bars, 200 μm.
Figure 3
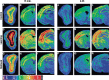
arg 3.1/arc mRNA expression in different brain areas detected by radioactive in situ hybridization. Pseudocolor images of film autoradiograms showing densities of arg 3.1/arc expression detected in different brain areas by in situ hybridization using a35S-labeled antisense probe. Control (A–C), learning (D–F), and retrieval (G–I) animals sacrificed 15 min after the test; control (J–L), learning (M–O) and retrieval (P–R) animals sacrificed 4.5 h after the test. Note the increased expression of arg 3.1/arc at 15 min compared with 4.5 h post-training in the three experimental groups, and in the learning group compared with the control and retrieval groups 15 min and 4.5 h after the session. (Gl) Glomerular layer of the olfactory bulb; (Gr) granule cell layer of the olfactory bulb; (ACC) anterior cingulate cortex; (M) motor cortex; (S) sensory cortex; (PCC) posterior cingulate cortex; (Par) parietal cortex; (CA1) CA1 subfield of the hippocampus; (CA): CA3 subfield of the hippocampus ; (DG) dentate gyrus of the hippocampus; (Pir) piriform cortex; (A) amygdala. Scale bars, 260 μm (A,D,G,J,M,P), 1370 μm (B,E,H,K,N,Q), 1060 μm (C,F,I,L,O,R).
Figure 4
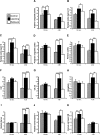
Quantification of arg 3.1/arc mRNA expression detected by radioactive in situ hybridization. Bar graphs illustrating the average relative optical density (+/− SEM) measured in several brain areas. Note the learning-induced peaks of arg 3.1/arc mRNA expression 15 min and 4.5 h post-training in the anterior cingulate cortex (A) and layers I–IV (B), and VI (C) of the parietal cortex, only at the 4.5-h time point in the posterior cingulate cortex (D), piriform cortex (E), hippocampus CA1 (F), CA2–CA3 (G), amygdala (H), dentate gyrus (I), layer V of the parietal cortex (J), and only 15 min post-training in the granule cell layer of the olfactory bulb (K). (*)P < 0.05; (**) P < 0.01; (***)P < 0.001.
Similar articles
- Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain.
Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Vazdarjanova A, et al. J Comp Neurol. 2006 Sep 20;498(3):317-29. doi: 10.1002/cne.21003. J Comp Neurol. 2006. PMID: 16871537 - Arc mRNA induction in striatal efferent neurons associated with response learning.
Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Daberkow DP, et al. Eur J Neurosci. 2007 Jul;26(1):228-41. doi: 10.1111/j.1460-9568.2007.05630.x. Eur J Neurosci. 2007. PMID: 17614950 - Arc/Arg3.1: linking gene expression to synaptic plasticity and memory.
Tzingounis AV, Nicoll RA. Tzingounis AV, et al. Neuron. 2006 Nov 9;52(3):403-7. doi: 10.1016/j.neuron.2006.10.016. Neuron. 2006. PMID: 17088207 Review. - Control of synaptic consolidation in the dentate gyrus: mechanisms, functions, and therapeutic implications.
Bramham CR. Bramham CR. Prog Brain Res. 2007;163:453-71. doi: 10.1016/S0079-6123(07)63025-8. Prog Brain Res. 2007. PMID: 17765733 Review.
Cited by
- The importance of having Arc: expression of the immediate-early gene Arc is required for hippocampus-dependent fear conditioning and blocked by NMDA receptor antagonism.
Czerniawski J, Ree F, Chia C, Ramamoorthi K, Kumata Y, Otto TA. Czerniawski J, et al. J Neurosci. 2011 Aug 3;31(31):11200-7. doi: 10.1523/JNEUROSCI.2211-11.2011. J Neurosci. 2011. PMID: 21813681 Free PMC article. - Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice.
Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Hein AM, et al. Brain Behav Immun. 2010 Feb;24(2):243-53. doi: 10.1016/j.bbi.2009.10.002. Epub 2009 Oct 13. Brain Behav Immun. 2010. PMID: 19825412 Free PMC article. - Alcoholic Extract of Ashwagandha Leaves Protects Against Amnesia by Regulation of Arc Function.
Gautam A, Kaul SC, Thakur MK. Gautam A, et al. Mol Neurobiol. 2016 Apr;53(3):1760-1769. doi: 10.1007/s12035-015-9117-2. Epub 2015 Mar 7. Mol Neurobiol. 2016. PMID: 25744565 - Arc/Arg3.1 mRNA global expression patterns elicited by memory recall in cerebral cortex differ for remote versus recent spatial memories.
Gusev PA, Gubin AN. Gusev PA, et al. Front Integr Neurosci. 2010 May 21;4:15. doi: 10.3389/fnint.2010.00015. eCollection 2010. Front Integr Neurosci. 2010. PMID: 20577636 Free PMC article. - Regulation of brain-derived neurotrophic factor-mediated transcription of the immediate early gene Arc by intracellular calcium and calmodulin.
Zheng F, Luo Y, Wang H. Zheng F, et al. J Neurosci Res. 2009 Feb;87(2):380-92. doi: 10.1002/jnr.21863. J Neurosci Res. 2009. PMID: 18798281 Free PMC article.
References
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: A role for the Locus Coeruleus. Science. 1996;274:1211–1215. - PubMed
- DiAntonio A. Translating activity into plasticity. Nature. 2000;405:1011–1012. - PubMed
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press, Inc.; 1997.
- Guthrie K, Rayhanabad J, Kuhl D, Gall C. Odors regulate Arc expression in neuronal ensembles engaged in odor processing. NeuroReport. 2000;11:1809–1813. - PubMed
- Guzowski JF, McNaughton BL, Barnes CA, Worley P. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical