Role of ESCRT-I in retroviral budding - PubMed (original) (raw)
Role of ESCRT-I in retroviral budding
Juan Martin-Serrano et al. J Virol. 2003 Apr.
Abstract
Retroviral late-budding (L) domains are required for the efficient release of nascent virions. The three known types of L domain, designated according to essential tetrapeptide motifs (PTAP, PPXY, or YPDL), each bind distinct cellular cofactors. We and others have demonstrated that recruitment of an ESCRT-I subunit, Tsg101, a component of the class E vacuolar protein sorting (VPS) machinery, is required for the budding of viruses, such as human immunodeficiency virus type 1 (HIV-1) and Ebola virus, that encode a PTAP-type L domain, but subsequent events remain undefined. In this study, we demonstrate that VPS28, a second component of ESCRT-I, binds to a sequence close to the Tsg101 C terminus and is therefore recruited to the plasma membrane by HIV-1 Gag. In addition, we show that Tsg101 exhibits a multimerization activity. Using a complementation assay in which Tsg101 is artificially recruited to sites of HIV-1 assembly, we demonstrate that the integrity of the VPS28 binding site within Tsg101 is required for particle budding. In addition, mutation of a putative leucine zipper or residues important for Tsg101 multimerization also impairs the ability of Tsg101 to support HIV-1 budding. A minimal multimerizing Tsg101 domain is a dominant negative inhibitor of PTAP-mediated HIV-1 budding but does not inhibit YPDL-type or PPXY-type L-domain function. Nevertheless, YDPL-type L-domain activity is inhibited by expression of a catalytically inactive mutant of the class E VPS ATPase VPS4. These results indicate that all three classes of retroviral L domains require a functioning class E VPS pathway in order to effect budding. However, the PTAP-type L domain appears to be unique in its requirement for an intact, or nearly intact, ESCRT-I complex.
Figures
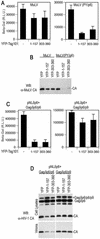
FIG. 1.
Domain organization of Tsg101. (A) Schematic representation of the 390-residue Tsg101 protein, showing the amino-terminal UBC-like, proline rich, leucine zipper (Leu-Z) and carboxy-terminal (C-Term) domains. The UBC-like domain is necessary and sufficient for PTAP binding, while the Leu-Z and C-Term domains are necessary and sufficient to mediate HIV-1 particle budding when artificially recruited to HIV-1 assembly sites. (B) Amino acid sequence of the Leu-Z and C-Term domains, showing mutations that were used in this study.
FIG. 2.
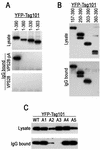
Mapping of a VPS28 binding site on Tsg101. (A) The indicated C-terminal Tsg101 deletion mutants were expressed in 293T cells as YFP fusion proteins along with either VPS28 or a VPS28-pA fusion protein. Cytoplasmic lysates (top panel) or proteins bound to IgG agarose beads (middle and lower panels) were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting with an anti-GFP antibody (B) N-terminally truncated Tsg101 proteins fused to YFP were coexpressed with VPS28-pA and analyzed as for panel A. (C) Mutant YFP-Tsg101 proteins A1 to A5 were coexpressed with VPS28-pA and analyzed for coprecipitation as for panels A and B.
FIG. 3.
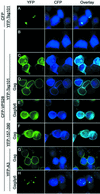
VPS28 is recruited to the plasma membrane as a result of HIV-1 p6Gag-Tsg101 interaction. 293T cells were transfected with YFP-Tsg101, CFP, CFP-VPS28, and/or HIV-1 Gag expression plasmids, fixed, and examined by deconvolution microscopy. The images represent single optical sections acquired with a YFP filter set (left column), a CFP filter set (middle column), and overlaid images (right column). (A) YFP-Tsg101 plus CFP. (B) CFP-VPS28 only. (C) YFP-Tsg101 plus CFP-VPS28. (D) YFP-Tsg101 plus CFP-VPS28 plus HIV-1 Gag. (E) YFP-Tsg101 plus CFP-VPS28 plus HIV-1 Gagδp6. (F) YFP-Tsg101(157-390) plus CFP-VPS28 plus HIV-1 Gag. (G) YFP-Tsg101(A3) plus CFP-VPS28 plus HIV-1 Gag. (H) YFP-Tsg101(A3) plus CFP-VPS28 plus HIV-1 Gagδp6.
FIG. 4.
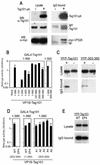
Tsg101 multimerization mediated by sequences in the leucine zipper and C-terminal domains. (A) 293T cells were transfected with a Myc-VPS28 expression plasmid alone (lanes −) or with both Tsg101-pA and Myc-VPS28 expression plasmids (lanes +). Cytoplasmic lysates (left panels) and IgG-bound protein complexes (right panels) were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting (WB) with anti-Tsg101 (α-Tsg101) (top and middle panels) or anti-Myc (lower panels) antibodies. (B) Yeast two-hybrid assay of Tsg101 multimerization. Y190 cells were transformed with GAL4-Tsg10(1-390) (full length) or GAL4-Tsg10(1-303) expression plasmids along with a plasmid expressing a full-length or deletion mutant VP16-Tsg101 expression plasmid, as indicated. The mean level of β-galactosidase expression (± standard deviation) in three pools of transformants is shown. (C) Coprecipitation of YFP-Tsg101 and Tsg101-pA. 293T cells were transfected with a YFP-Tsg101 (left panels) or a YFP-Tsg101(303-360) (right panels) expression plasmid in the presence (lanes +) or absence (lanes −) of a Tsg101-pA expression plasmid. Cell lysates (top panels) or IgG-bound protein complexes (bottom panels) were analyzed by Western blotting with an anti-GFP antibody. (D) Yeast two-hybrid analysis of mutant Tsg101 protein multimerization. Y190 cells were transformed with GAL4-Tsg101 along with a plasmid expressing a full-length or mutant VP16-Tsg101. The mean level of β-galactosidase expression (± standard deviation) in three pools of transformants is shown. (E) 293T cells were transfected with full-length YFP-Tsg101 (wild type [WT]) or YFP-Tsg101(EP4) and VPS28-pA expression plasmids. Cell lysates (top panel) or IgG-bound protein complexes (bottom panel) were analyzed by Western blotting with an anti-GFP antibody.
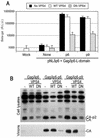
FIG. 5.
Leucine zipper, multimerization, and VPS28 binding domains of Tsg101 are required to mediate HIV-1 virion budding. (A) Y190 yeast cells were transformed with plasmids expressing wild-type (WT) or leucine zipper mutant GAL4-Tsg101 proteins as well as VP16-HIV-1 Gag, VP16-Tsg101, or VP16-VPS28, as indicated. The mean level of β-galactosidase (β-Gal) expression (± standard deviation) in three pools of transformants is shown. (B) HOS cells were transfected with an L-domain-defective HIV-1 proviral plasmid, pNL(LTAL), and a complementing expression vector. The complementing vector expressed a Gagδp6 protein alone (lane −) or a Gagδp6 protein to which either p6 or wild-type or mutant forms of Tsg101 were fused at the C terminus. Cell lysates and extracellular particles were analyzed by Western blotting with an anti-HIV-1 CA antibody. (C) Culture supernatants from HOS cells transfected as for panel B were analyzed for the presence of infectious virions by inoculation of P4/R5 indicator cells. β-Galactosidase activity in cell lysates was measured 48 h after inoculation, and the mean and standard deviation are shown. The background β-galactosidase activity in lysates of uninfected P4/R5 cells (Un) was approximately 1,000 relative light units (R.L.U.).
FIG. 6.
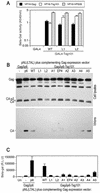
Selective inhibition of PTAP-type L-domain function by a minimal Tsg101 multimerizing domain. (A) 293T cells were transfected with an MuLV proviral plasmid and an MuLV-based HIV-1 Tat expression vector along with a YFP, YFP-Tsg101(1-157), or YFP-Tsg101(303-360) expression plasmid. The MuLV provirus harbored either the endogenous PPXY type L domain (left panel) or a PTAP motif derived from HIV-1 (right panel). Infectious MuLV virion production was measured by inoculation of P4/R5 indicator cells, and β-galactosidase (β-Gal) activity in lysates was measured 48 h later. The mean and standard deviation are shown, and the background β-galactosidase activity in lysates of uninfected P4/R5 cells was approximately 1,000 relative light units (R.L.U.). (B) Western blot (WB) analysis of extracellular MuLV virion production by 293T cells transfected with the same constructs as for panel A, using an anti-MuLV (α-MuLV) CA antibody. (C) 293T cells were transfected with an L-domain-defective HIV-1 proviral plasmid, (pNLδp6) and a complementing and Gagδp6 expression vector along with a YFP, YFP-Tsg101(1-157), or YFP-Tsg101(303-360) expression plasmid. The complementing Gagδp6 vector expressed either HIV-1 p6 (left panel) or EIAV p9 (right panel) fused at its C terminus. Infectious HIV-1 virion production was measured by inoculation of P4/R5 indicator cells, and β-galactosidase activity in lysates was measured 48 h later. The mean and standard deviation are shown. (D) Western blot analysis of cell lysates and extracellular HIV-1 virion production by 293T cells transfected with the same constructs as for panel C, using an anti-HIV-1 CA antibody.
FIG. 7.
EIAV YPDL-type L-domain activity is inhibited by catalytically inactive VPS4. (A) 293T cells were transfected with an L-domain-defective HIV-1 proviral plasmid, (pNLδp6) and a complementing Gagδp6 expression vector that expressed either no L domain (None), HIV-1 p6, or EIAV p9 fused at its C terminus, as indicated. Either no VPS4 or wild-type (WT) or DN mutant forms of VPS4 were coexpressed. Infectious HIV-1 virion production was measured by inoculation of P4/R5 indicator cells, and β-galactosidase (β-Gal) activity in lysates was measured 48 h later. The mean and standard deviation are shown. R.L.U., relative light units. (B) Western blot analysis of cell lysates and extracellular HIV-1 virion production by 293T cells transfected with the same constructs as for panel A, using an anti-HIV-1 CA antibody.
Similar articles
- Identification of human VPS37C, a component of endosomal sorting complex required for transport-I important for viral budding.
Eastman SW, Martin-Serrano J, Chung W, Zang T, Bieniasz PD. Eastman SW, et al. J Biol Chem. 2005 Jan 7;280(1):628-36. doi: 10.1074/jbc.M410384200. Epub 2004 Oct 27. J Biol Chem. 2005. PMID: 15509564 - Defects in human immunodeficiency virus budding and endosomal sorting induced by TSG101 overexpression.
Goila-Gaur R, Demirov DG, Orenstein JM, Ono A, Freed EO. Goila-Gaur R, et al. J Virol. 2003 Jun;77(11):6507-19. doi: 10.1128/jvi.77.11.6507-6519.2003. J Virol. 2003. PMID: 12743307 Free PMC article. - The human endosomal sorting complex required for transport (ESCRT-I) and its role in HIV-1 budding.
Stuchell MD, Garrus JE, Müller B, Stray KM, Ghaffarian S, McKinnon R, Kräusslich HG, Morham SG, Sundquist WI. Stuchell MD, et al. J Biol Chem. 2004 Aug 20;279(34):36059-71. doi: 10.1074/jbc.M405226200. Epub 2004 Jun 23. J Biol Chem. 2004. PMID: 15218037 - Novel Tsg101 Binding Partners Regulate Viral L Domain Trafficking.
Strickland M, Nyenhuis D, Watanabe SM, Tjandra N, Carter CA. Strickland M, et al. Viruses. 2021 Jun 15;13(6):1147. doi: 10.3390/v13061147. Viruses. 2021. PMID: 34203832 Free PMC article. Review. - [HIV budding and Tsg101].
Yasuda J. Yasuda J. Uirusu. 2005 Dec;55(2):281-6. doi: 10.2222/jsv.55.281. Uirusu. 2005. PMID: 16557014 Review. Japanese.
Cited by
- Transduction Efficiency of Zika Virus E Protein Pseudotyped HIV-1_gfp_ and Its Oncolytic Activity Tested in Primary Glioblastoma Cell Cultures.
Formanski JP, Ngo HD, Grunwald V, Pöhlking C, Jonas JS, Wohlers D, Schwalbe B, Schreiber M. Formanski JP, et al. Cancers (Basel). 2024 Feb 17;16(4):814. doi: 10.3390/cancers16040814. Cancers (Basel). 2024. PMID: 38398205 Free PMC article. - Effects of Chemokine Ligand 2 on Budding of Bovine Foamy Virus.
Li R, Wang Z, Liu C, Qiao W, Tan J. Li R, et al. Viruses. 2023 Sep 1;15(9):1867. doi: 10.3390/v15091867. Viruses. 2023. PMID: 37766274 Free PMC article. - HIV-1 promotes ubiquitination of the amyloidogenic C-terminal fragment of APP to support viral replication.
Gu F, Boisjoli M, Naghavi MH. Gu F, et al. Nat Commun. 2023 Jul 15;14(1):4227. doi: 10.1038/s41467-023-40000-x. Nat Commun. 2023. PMID: 37454116 Free PMC article. - Snf7 spirals sense and alter membrane curvature.
Jukic N, Perrino AP, Humbert F, Roux A, Scheuring S. Jukic N, et al. Nat Commun. 2022 Apr 21;13(1):2174. doi: 10.1038/s41467-022-29850-z. Nat Commun. 2022. PMID: 35449207 Free PMC article. - Characterization of Bovine Foamy Virus Gag Late Assembly Domain Motifs and Their Role in Recruiting ESCRT for Budding.
Wang Z, Li R, Liu C, Qiao W, Tan J. Wang Z, et al. Viruses. 2022 Mar 3;14(3):522. doi: 10.3390/v14030522. Viruses. 2022. PMID: 35336929 Free PMC article.
References
- Babst, M., D. Katzmann, E. Estepa-Sabal, T. Meerloo, and S. Emr. 2002. Escrt-III. An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3:271.. - PubMed
- Babst, M., D. Katzmann, W. Snyder, B. Wendland, and S. Emr. 2002. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3:283.. - PubMed
- Babst, M., G. Odorizzi, E. J. Estepa, and S. D. Emr. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1:248-258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources