A GTP-driven motor moves proteins across the outer envelope of chloroplasts - PubMed (original) (raw)
A GTP-driven motor moves proteins across the outer envelope of chloroplasts
Enrico Schleiff et al. Proc Natl Acad Sci U S A. 2003.
Abstract
The translocation of proteins across cellular membranes is a key mechanistic problem for every cell. The preprotein translocon at the chloroplast outer envelope is responsible for precursor protein recognition and translocation across the outer envelope. We have reconstituted the translocation process into proteoliposomes from single subunits or by using the purified translocon. Precursor proteins are recognized by the Toc34 receptor in an initial GTP-dependent process. Translocation across the plane of the membrane then occurs through the Toc75 channel in a GTP-dependent process. Correspondingly, GTP hydrolysis of Toc proteoliposomes is 100-fold enhanced in the presence of preprotein. Complete translocation is demonstrated by processing of the precursor form to the mature form by the stromal processing peptidase and by protease resistance of the imported protein. Molecular chaperones are not involved in this translocation event. We show that Toc159 acts as a GTP-driven motor in a sewing-machine-like mechanism.
Figures
Figure 1
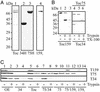
Reconstitution of the subunits of the Toc core complex. (A) The purified Toc complex (Toc) was controlled by SDS/PAGE followed by silver staining (lane 1). Purity of heterologously expressed Toc34 (34H) and Toc75 (75H) containing C-terminal hexa-histidine tags and eluted Toc159f (159f) was controlled by SDS/PAGE followed by Coomassie blue staining (lanes 2, 3, and 4). (B) The isolated Toc complex was reconstituted into liposomes, diluted to a 2-mM final lipid concentration (B, lanes 1 and 4; C, lane 5), and incubated with 25 ng of trypsin for 5 min at 25°C in a volume of 100 μl in the absence (B, lanes 2 and 5; C, lane 6) or presence (B, lanes 3 and 6) of 0.1% Triton X-100. Reconstitution and proteolysis were followed by immunodecoration by using antibodies against Toc159 (B Left; C, αToc159), Toc34 (B Lower Right; C, αToc34) and Toc75 (B Upper Right; C, αToc75). (C) Toc34 (lanes 3 and 4), Toc75 (lanes 9 and 10), Toc34/Toc75 (lanes 7 and 8), Toc159f/Toc75 (lanes 11 and 12), and Toc159f (lanes 13 and 14) were reconstituted (lanes 3, 7, 9, 11, and 13), and protease resistance was tested (lanes 4, 8, 10, 12, and 14) by the procedure used for B. For comparison, outer-envelope vesicles (lane 1) were also treated with trypsin (lane 2).
Figure 2
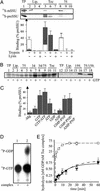
Biological activity of the Toc proteoliposomes. (A) 35S-labeled mSSU (Top) and preSSU (Middle) were incubated with empty liposomes (lanes 2–4) or proteoliposomes (lanes 5–10; 1-mM final lipid concentration; nomenclature as in Fig. 1) in the presence of 1 mM GTP (lanes 2–10) followed by competition with N-liposomes. Proteoliposomes were diluted to 1-mM final lipid concentration and incubated with 10 ng of trypsin for 5 min at 25°C in a volume of 100 μl in the absence (lanes 3, 6, and 9) or presence (lanes 4, 7, and 10) of 0.1% Triton X-100. One hundred percent of the translation product used is shown for comparison (lane 1). The binding of preSSU (gray bar) and mSSU (white bar) of at least three independent experiments was quantified and compared with the amount of translation product used. (B) Binding of [35S]methionine-labeled preSSU to empty (lanes 2, 3, 13, and 14) or proteoliposomes (lanes 4–11 and 15–18; nomenclatures in Fig. 1) was performed in the absence (lanes 2, 4, 6, 8, 10, 13, 15, and 17) or presence (lanes 3, 5, 7, 9, 11, 14, 16, and 18) of 0.5 mM MgCl2/1 mM GTP. Ten percent of used translation product is shown for comparison (lanes 1 and 12). (C) Binding of 35S-labeled preSSU to Toc proteoliposomes was initiated in the absence (−Mg) or presence of 0.5 mM MgCl2 and 1 mM of the indicated nucleotide. The SE of at least three independent experiments are indicated. (D) The hydrolysis of 330 nM radiolabeled [α-32P]GTP in the absence (lane 1) or presence (lane 2) of the purified Toc complex (5-nM final concentration) was performed. (E) The amount of hydrolyzed GTP at time points indicated are means of at least three independent experiments in the absence (E, ●, solid line) or presence of 100 nM purified mSSU (E, ▵, dash-dot-dot line) or preSSU (E, □, dashed line).
Figure 3
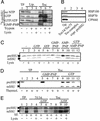
GTP drives protein translocation through Toc complex. (A) 35S-labeled preSSU was incubated with empty liposomes (lanes 2–4) or proteoliposomes (lanes 5–7, 1 mM final lipid concentration; nomenclature as in Fig. 1) in the presence of 1 mM nucleotide. After competition, proteoliposomes were proteolyzed by trypsin in the absence (lanes 3 and 5) or presence (lanes 4, 7, and 10) of 0.1% Triton X-100. Five percent of the translation product used is shown (lane 1). (B) Outer envelope membranes (lane 1), proteoliposomes containing Toc34 and Toc75 (lane 2) or the Toc complex (lane 3), or the purified radiolabeled preSSU (RTS; lane 4) were subjected to SDS/PAGE followed by immunodecoration by using antibodies against HSP100 (Top), HSP70 (Middle), and CPN60 (Bottom). (C) Binding of the purified preSSU (RTS) to the proteoliposomes containing stromal extract and apyrase (lanes 1–12) was initiated in the presence of 1 mM of the indicated nucleotide. After competition with N-liposomes, liposomes were dissolved by 0.1% Triton X-100 (lanes 2, 4, 6, 8, 10, and 12) and further incubated for 30 min at 25°C. (D) Toc proteoliposomes containing stromal extract and apyrase were incubated with preSSU (RTS) in the presence of 1 mM GMP-PNP (lanes 5–8) or 1 mM GTP (lanes 9 and 10; see C for details). After final incubation for 30 min at 25°C in 100 μl, proteolysis by thermolysin (4 μg/ml final) at 4°C was initiated for 5 (lanes 2, 6, and 10), 10 (lanes 3, 7, and 11), and 20 min (lanes 4, 8, and 12) and stopped by excess of EDTA/EGTA. In lanes 1–4, 50% of the used preSSU was treated as described above. (E) Liposomes were loaded with stromal extract, apyrase, and 150 mM poly-
l
-glutamic acid/poly-
l
-aspartic acid. The binding of _in vitro_-translated preSSU to proteoliposomes was performed in the absence (lanes 3, 4, 7, and 8) or presence (lanes 5, 6, 9, and 10) of 1 mM GTP. For comparison, translation product (lane 1) was incubated for 30 min at 25°C with stromal extract (lane 2).
Figure 4
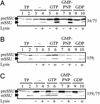
Toc159f is sufficient to transport a precursor through the Toc75 pore in a GTP-dependent manner. Toc34/Toc75 (A), Toc159f (B), and Toc159f/Toc75 (C) were reconstituted into liposomes. The different proteoliposomes containing stromal extract and apyrase were then incubated with purified preSSU (RTS; lanes 3–10) in the presence of 1 mM of the indicated nucleotide. After competition with N-liposomes, liposomes were either not dissolved (lanes 3, 5, 7, and 9) or dissolved by 0.1% Triton X-100 (lanes 4, 6, 8, and 10) and further incubated for 30 min at 25°C. For control, 10% of the purified protein used (lane 1) was incubated with stromal extract (lane 2).
Figure 5
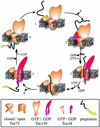
Model of the translocation process at the outer envelope of chloroplasts. Import is initiated by GTP-dependent recognition of the precursor protein by Toc34 (step 1). GTP hydrolysis by Toc34 induces heterodimerization and transfer of the precursor toward Toc159 (step 2). The subsequent hydrolysis of GTP induces a structural change within the receptor, pushing the precursor into or across the translocation channel (step 3). GDP–GTP exchange of Toc159 might then cause the relaxation (step 4) and initiation of a next round of translocation (step 5), eventually resulting in complete translocation (step 6).
Similar articles
- The roles of toc34 and toc75 in targeting the toc159 preprotein receptor to chloroplasts.
Wallas TR, Smith MD, Sanchez-Nieto S, Schnell DJ. Wallas TR, et al. J Biol Chem. 2003 Nov 7;278(45):44289-97. doi: 10.1074/jbc.M307873200. Epub 2003 Sep 1. J Biol Chem. 2003. PMID: 12951325 - Toc34 is a preprotein receptor regulated by GTP and phosphorylation.
Sveshnikova N, Soll J, Schleiff E. Sveshnikova N, et al. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4973-8. doi: 10.1073/pnas.080491597. Proc Natl Acad Sci U S A. 2000. PMID: 10781107 Free PMC article. - The targeting of the atToc159 preprotein receptor to the chloroplast outer membrane is mediated by its GTPase domain and is regulated by GTP.
Smith MD, Hiltbrunner A, Kessler F, Schnell DJ. Smith MD, et al. J Cell Biol. 2002 Dec 9;159(5):833-43. doi: 10.1083/jcb.200208017. Epub 2002 Dec 9. J Cell Biol. 2002. PMID: 12473690 Free PMC article. - Protein transport in organelles: The Toc complex way of preprotein import.
Agne B, Kessler F. Agne B, et al. FEBS J. 2009 Mar;276(5):1156-65. doi: 10.1111/j.1742-4658.2009.06873.x. FEBS J. 2009. PMID: 19187236 Review. - New insights into the mechanism of chloroplast protein import and its integration with protein quality control, organelle biogenesis and development.
Paila YD, Richardson LGL, Schnell DJ. Paila YD, et al. J Mol Biol. 2015 Mar 13;427(5):1038-1060. doi: 10.1016/j.jmb.2014.08.016. Epub 2014 Aug 28. J Mol Biol. 2015. PMID: 25174336 Free PMC article. Review.
Cited by
- The molecular chaperone Hsp90 delivers precursor proteins to the chloroplast import receptor Toc64.
Qbadou S, Becker T, Mirus O, Tews I, Soll J, Schleiff E. Qbadou S, et al. EMBO J. 2006 May 3;25(9):1836-47. doi: 10.1038/sj.emboj.7601091. Epub 2006 Apr 13. EMBO J. 2006. PMID: 16619024 Free PMC article. - Toc12, a novel subunit of the intermembrane space preprotein translocon of chloroplasts.
Becker T, Hritz J, Vogel M, Caliebe A, Bukau B, Soll J, Schleiff E. Becker T, et al. Mol Biol Cell. 2004 Nov;15(11):5130-44. doi: 10.1091/mbc.e04-05-0405. Epub 2004 Aug 18. Mol Biol Cell. 2004. PMID: 15317846 Free PMC article. - Chloroplast biogenesis: control of plastid development, protein import, division and inheritance.
Sakamoto W, Miyagishima SY, Jarvis P. Sakamoto W, et al. Arabidopsis Book. 2008;6:e0110. doi: 10.1199/tab.0110. Epub 2008 Jul 22. Arabidopsis Book. 2008. PMID: 22303235 Free PMC article. - The acidic domains of the Toc159 chloroplast preprotein receptor family are intrinsically disordered protein domains.
Richardson LG, Jelokhani-Niaraki M, Smith MD. Richardson LG, et al. BMC Biochem. 2009 Dec 30;10:35. doi: 10.1186/1471-2091-10-35. BMC Biochem. 2009. PMID: 20042108 Free PMC article. - atToc159 is a selective transit peptide receptor for the import of nucleus-encoded chloroplast proteins.
Smith MD, Rounds CM, Wang F, Chen K, Afitlhile M, Schnell DJ. Smith MD, et al. J Cell Biol. 2004 May 10;165(3):323-34. doi: 10.1083/jcb.200311074. J Cell Biol. 2004. PMID: 15138290 Free PMC article.
References
- Cavalier-Smith T. Trends Plant Sci. 2000;5:174–182. - PubMed
- Chen X, Schnell D J. Trends Cell Biol. 1999;9:222–227. - PubMed
- Schleiff E, Soll J. Planta. 2000;211:449–456. - PubMed
- Kessler F, Blobel G, Patel H A, Schnell D J. Science. 1994;266:1035–1039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources