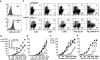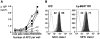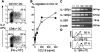Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells - PubMed (original) (raw)
Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells
Anne Krug et al. J Exp Med. 2003.
Abstract
Interferon-producing cells (IPCs) secrete high levels of type I interferon in response to certain viruses. The lack of lineage markers, the expression of major histocompatibility complex (MHC) class II and the capacity to stimulate allogeneic T cells have led these cells to be classified as a subset of dendritic cells (DCs), called plasmacytoid DCs (PDCs). However, the role of IPCs/PDCs in initiating primary immune responses remains elusive. Here we examined the antigen presenting capacity of murine IPCs in antigen specific systems. While CD8alpha+ and CD11b+ DCs induced logarithmic expansion of naive CD4 and CD8 T cells, without conferring T helper commitment at a first encounter, primary IPCs lacked the ability to stimulate naive T cells. However, when antigen-experienced, nonpolarized T cells expanded by classical DC subsets, were restimulated by IPCs, they proliferated and produced high amounts of IFN-gamma. These data indicate that IPCs can effectively stimulate preactivated or memory-type T cells and exert an immune-regulatory role. They also suggest that expansion of naive T cells and acquisition of effector function during antigen-specific T cell responses may involve different antigen-presenting cell (APC) types. Independent and coordinated control of T cell proliferation and differentiation would provide the immune system with greater flexibility in regulating immune responses.
Figures
Figure 1.
HEL expression and antigen presentation to HEL-specific naive CD4 T cells and 3A9 T cell hybridoma by primary mHEL-transgenic DCs and IPCs. (A) IPCs and Ly-6G/C− DCs from the mHEL mouse were stained with an antibody specific for HEL to measure antigen expression at the cell surface (gray histograms). Open histograms indicate staining with control antibody. (B) Proliferative response of HEL-specific naive 3A9 CD4 T cells (104) cultured with graded numbers of purified IPCs (open symbols), CD11b+(black filled symbols), and CD8α+ (gray filled symbols) DCs derived from mHEL mice. IPCs and DCs were untreated (circles) or treated (squares) in vitro for 12 h with PR8 (1 MOI) before addition of T cells. Supernatants were then collected and type I IFN production was measured (insert to B). Proliferation was measured after 72 h. (C) Expression of costimulatory molecules, MHC class II and HEL48–62/IAk specific complexes in IPCs and DCs derived from mHEL-mice. Mice were either uninfected or challenged with VSV for 12 h. MFI for IPCs and Ly-6G/C− DCs are indicated. (D) Proliferation of 3A9 naive CD4 T cells cocultured with graded numbers of IPCs or DCs purified ex vivo from mHEL mice uninfected or infected with VSV (intravenous, 2 × 108 PFU) 12 h before. (E and F) HEL-specific 3A9 cell hybridoma T cells (105) were cocultured with graded numbers of IPCs and DCs sorted from mHEL mice and stimulated in vitro with influenza virus PR8 (E) or activated in vivo by VSV infection (F). Supernatants were collected after 48 h and the IL-2 produced upon activation was measured as proliferation of CTLL cells after 20 h.
Figure 2.
Antigen presentation to HY-specific naive CD8 T cells by HY-transgenic IPCs and DCs. (A) Proliferation of naive CD8 T cells (5 × 104) purified from HY-transgenic mice to IPCs (open symbols) or DCs (filled symbols) derived from uninfected or VSV infected H-2Db B6 males. VSV infection was performed as in Fig. 1. Proliferation was measured after 72 h. (B) Expression of H-2Db class I molecules in IPCs or DCs of uninfected mice (light gray histograms) or VSV infected mice (dark gray histograms). Open histograms show staining with a control antibody. Numbers indicate MFI.
Figure 3.
DCs induce expansion of antigen experienced uncommitted T cells which have lymph node homing potential and can respond to IFN-γ–inducing cyto-kines. (A) CD4+ 3A9 T cells expanded by freshly isolated CD11b+ or CD8α+ mHEL mouse-derived DCs were analyzed for IFN-γ and IL-2 production, 5–7 d after the first stimulation. (B) In vitro migration to CCL19 of 3A9 T cells expanded by CD11b+ DCs (black filled symbols) or CD8α+ DCs (gray filled symbols) is dose dependent. (C and D) 3A9 T cells expanded by CD11b+ DCs express IL-12, IL-23, and IL-18 receptors thus having the capacity to respond to all these IFN-γ–inducing cytokines. IL-12Rβ1, IL-12Rβ2, IL-23R, and β-actin were detected by RT-PCR. (C) Numbers indicate fold dilutions of cDNAs. IL-12Rβ (β1 and β2 chains), and IL18Rα chain expression were measured at the cell surface of 3A9 T cells expanded by CD11b+ DCs by flow cytometry at the same time of restimulation. Identical results were obtained with 3A9 T cells expanded by CD8α+ DCs.
Figure 4.
IPCs induce proliferation and IFN-γ production by antigen experienced unpolarized T cells. (A) Proliferative response of 3A9 T cells expanded by CD11b+ DCs to graded numbers of primary unstimulated DCs, IPCs, or B cells. 3A9 T cells were restimulated 9–10 d after the first stimulation. (B) IFN-γ and IL-4 production in 3A9 T cells restimulated with primary unstimulated IPCs, CD8α+, and CD11b+ DCs. The percentage of positive cells and mean fluorescence intensity of IFN-γ–producing cells are indicated. (C) Quantitation of IL-12p70 in supernatants of T cell-DC and T cell–IPC cocultures. Supernatants were collected after 24 h and IL-12p70 was measured by ELISA.
Figure 5.
Th1 polarization induced by IPCs is IL-12 independent and only partially reverted by neutralizing antibodies to IFN-β and IL-18. (A) IFN-γ and IL-4 production in 3A9 T cells restimulated with freshly isolated unstimulated IPCs or CD8α+ DCs in the absence or in the presence of neutralizing antibodies to IFN-β and IL-18Rα and/or IL-12. The concomitant neutralization of IFN-β and IL-18 slightly reduce the level of IFN-γ production in T cells restimulated with IPCs; neutralization of IL-12 greatly reduced the number of IFN-γ–producing cells in T cells restimulated by CD8α+ DCs. The percentage of positive cells and mean fluorescence intensity of IFN-γ–producing cells are indicated. (B) Expression of IL-23p19, IL-12p35, and IL-12/IL-23p40 common subunit in freshly isolated and CpG (ODN 1826) stimulated IPCs and DCs measured by RT-PCR. Induction of p40 and p19 can be observed in stimulated IPCs.
Similar articles
- Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy.
Feili-Hariri M, Falkner DH, Morel PA. Feili-Hariri M, et al. J Leukoc Biol. 2005 Sep;78(3):656-64. doi: 10.1189/jlb.1104631. Epub 2005 Jun 16. J Leukoc Biol. 2005. PMID: 15961574 - Dendritic cell lineage, plasticity and cross-regulation.
Liu YJ, Kanzler H, Soumelis V, Gilliet M. Liu YJ, et al. Nat Immunol. 2001 Jul;2(7):585-9. doi: 10.1038/89726. Nat Immunol. 2001. PMID: 11429541 Review. - IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors.
Liu YJ. Liu YJ. Annu Rev Immunol. 2005;23:275-306. doi: 10.1146/annurev.immunol.23.021704.115633. Annu Rev Immunol. 2005. PMID: 15771572 Review.
Cited by
- CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens.
Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. Salio M, et al. J Exp Med. 2004 Feb 16;199(4):567-79. doi: 10.1084/jem.20031059. J Exp Med. 2004. PMID: 14970182 Free PMC article. - Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells.
Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Sapoznikov A, et al. J Exp Med. 2007 Aug 6;204(8):1923-33. doi: 10.1084/jem.20062373. Epub 2007 Jul 23. J Exp Med. 2007. PMID: 17646404 Free PMC article. - Tacrolimus treatment of plasmacytoid dendritic cells inhibits dinucleotide (CpG-)-induced tumour necrosis factor-alpha secretion.
Naranjo-Gómez M, Climent N, Cos J, Oliva H, Bofill M, Gatell JM, Gallart T, Pujol-Borrell R, Borràs FE. Naranjo-Gómez M, et al. Immunology. 2006 Dec;119(4):488-98. doi: 10.1111/j.1365-2567.2006.02460.x. Epub 2006 Aug 24. Immunology. 2006. PMID: 16930148 Free PMC article. - Plasmacytoid dendritic cells take up opsonized antigen leading to CD4+ and CD8+ T cell activation in vivo.
Björck P, Beilhack A, Herman EI, Negrin RS, Engleman EG. Björck P, et al. J Immunol. 2008 Sep 15;181(6):3811-7. doi: 10.4049/jimmunol.181.6.3811. J Immunol. 2008. PMID: 18768834 Free PMC article. - Vaccinia virus infection modulates the hematopoietic cell compartments in the bone marrow.
Singh P, Yao Y, Weliver A, Broxmeyer HE, Hong SC, Chang CH. Singh P, et al. Stem Cells. 2008 Apr;26(4):1009-16. doi: 10.1634/stemcells.2007-0461. Epub 2008 Feb 7. Stem Cells. 2008. PMID: 18258722 Free PMC article.
References
- Liu, Y.J., H. Kanzler, V. Soumelis, and M. Gilliet. 2001. Dendritic cell lineage, plasticity and cross-regulation. Nat. Immunol. 2:585–589. - PubMed
- Colonna, M., A. Krug, and M. Cella. 2002. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14:373–379. - PubMed
- Siegal, F.P., N. Kadowaki, M. Shodell, P.A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y.J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science. 284:1835–1837. - PubMed
- Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919–923. - PubMed
- Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305–310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials