An essential role for hGle1 nucleocytoplasmic shuttling in mRNA export - PubMed (original) (raw)
An essential role for hGle1 nucleocytoplasmic shuttling in mRNA export
Frederic Kendirgi et al. J Cell Biol. 2003.
Abstract
Gle1 is required for mRNA export in yeast and human cells. Here, we report that two human Gle1 (hGle1) isoforms are expressed in HeLa cells (hGle1A and B). The two encoded proteins are identical except for their COOH-terminal regions. hGle1A ends with a unique four-amino acid segment, whereas hGle1B has a COOH-terminal 43-amino acid span. Only hGle1B, the more abundant isoform, localizes to the nuclear envelope (NE) and pore complex. To test whether hGle1 is a dynamic shuttling transport factor, we microinjected HeLa cells with recombinant hGle1 and conducted photobleaching studies of live HeLa cells expressing EGFP-hGle1. Both strategies show that hGle1 shuttles between the nucleus and cytoplasm. An internal 39-amino acid domain is necessary and sufficient for mediating nucleocytoplasmic transport. Using a cell-permeable peptide strategy, we document a role for hGle1 shuttling in mRNA export. An hGle1 shuttling domain (SD) peptide impairs the export of both total poly(A)+ RNA and the specific dihydrofolate reductase mRNA. Coincidentally, SD peptide-treated cells show decreased endogenous hGle1 localization at the NE and reduced nucleocytoplasmic shuttling of microinjected, recombinant hGle1. These findings pinpoint the first functional motif in hGle1 and link hGle1 to the dynamic mRNA export mechanism.
Figures
Figure 1.
hGle1 encodes two mRNA isoforms in HeLa cells. (A) Intracellular localization of endogenous hGle1 (top; using anti-hGle1 antibodies; Watkins et al., 1998), ectopically expressed EGFP–hGle1A (middle), and EGFP–hGle1B (bottom) in fixed HeLa cells (Fix-Triton; see Materials and methods). Double IIF with mAb414 was used to detect NPC/NE localization. Bar, 10 μm. (B) Schematic representation of hGle1. Exons are boxed, numbered 5′ to 3′, with nos. 15 and 16 specific to hGle1B (gray boxes). Primers used to specifically amplify hGle1A and hGle1B in RT-PCR experiments are F (common forward primer), A (hGle1A 3′ UTR–specific reverse primer), and B (hGle1B-specific reverse primer). (C) Semiquantitative RT-PCR analysis of hGle1A and hGleB mRNA levels in HeLa cells. Serial dilutions of total HeLa cell cDNA were tested using primer combinations F/A and F/B for hGle1A and hGle1B, respectively. Amplification products (202 bp and 180 bp, respectively) were separated on agarose gel and stained with ethidium bromide. Similar intensities at cDNA dilutions of 10−2 for hGle1A and 10−5 for hGle1B (arrowheads) suggest that hGle1B mRNA is 103-fold more abundant. (D) Sequence comparison (ClustalW) of the COOH-terminal regions of scGle1, hGle1A, and hGle1B (Pearson and Lipman, 1988). Boxed region 444–483 of hGle1A/B harbors shuttling activity. The putative LR-NES in scGle1 is double underlined. The unique four amino acids of hGle1A are underlined. *, identical residues; : or ., conserved residues.
Figure 2.
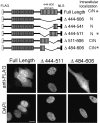
Delineating the hGle1 domain with nuclear export activity. (Top) Schematic representation and summary of the intracellular localizations for FLAG–hGle1A–NLS deletion constructs. C, cytoplasmic localization; N, nuclear localization; *, representative localization below. (Bottom) IIF localization of representative FLAG–hGle1–NLS proteins after transient expression in HeLa cells. Anti-FLAG monoclonal antibodies (top row) and staining for nuclear DNA with DAPI (bottom row) are shown. The cells in each figure are representative of the localization seen in the majority (∼70%; n = 500) of the cells across the total population of transfectants. Bar, 10 μm.
Figure 3.
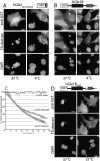
Analysis of GST–hGle1 dynamics in HeLa cells by microinjection. (A) The hGle1 39–amino acid region from 444–483 has intrinsic nucleocytoplasmic shuttling activity. HeLa cells were comicroinjected with purified proteins and TR-labeled 70-kD dextran. Cells were incubated for 5 h at 37°C or 4°C after injection and processed for IIF microscopy using anti-GST antibodies (top). Nuclear DNA was stained with DAPI (bottom). At 37°C (left), microinjection of GST–hGle1444–483 (SD) in one nucleus of a binucleate HeLa cell (top, arrow), as indicated by the TR-labeled dextran (middle), results in GST staining in the second nucleus (arrowhead). At 4°C (right), nuclear export and import activity of GST–hGle1444–483 (SD) is not detected. (arrow, nuclear injection; asterisk, cytoplasmic injection). (B and D) The SD is necessary for GST–hGle1B export. Full-length GST–hGle1B (B) and GST–hGle1ΔSD (D) were analyzed by microinjection. (B) After injection into one nucleus of a binucleate cell (arrowhead), GST–hGle1B is in both the cytoplasm and the uninjected nucleus after incubation at 37°C (right). Cytoplasmically microinjected GST–hGle1B (asterisk) shows strong nuclear staining. At 4°C (left), injected protein remains at the site of microinjection. (C) FLIP analysis reveals that EGFP–hGle1B shuttles between the nucleus and cytoplasm in HeLa cells. An area of the cytoplasm was repeatedly bleached, and the loss of nuclear fluorescence was monitored over time. The data points plotted for EGFP–hGle1B represent averages (n = 6). EGFP–coilin and EGFP–hNup98 data are representative of the loss of fluorescence detected in several time courses and are consistent with data previously reported (Griffis et al., 2002). (D) Microinjection of GST–hGle1BΔSD protein in the nucleus results in no staining outside the microinjection site after incubation at 37°C (both panels) (or 4°C; not depicted). In the right panel, partial nuclear localization is observed in two cytoplasmically injected cells (asterisks). Bars, 10 μm.
Figure 4.
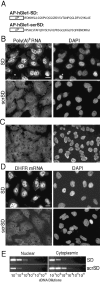
The AP–hGle1-SD peptide results in nuclear poly(A) + RNA accumulation. (A) Schematic representation of the cell-permeable peptides used. AP–hGle1-SD, antennapedia 16–amino acid leader peptide (AP) followed by hGle1 amino acids 444–483 (hGle1-SD) or followed by randomly scrambled sequence of identical SD composition (AP–hGle1-scrSD). (B) In situ hybridization experiments detect nuclear poly(A)+ RNA accumulation in AP–hGle1-SD–treated cells. HeLa cells (<10 passages) were incubated for 4 h with 5 μM of AP–hGle1-SD (SD) or the control AP–hGle1-scrSD peptide (scrSD) in normal growth medium. In situ hybridization using digoxigenin-labeled oligo(dT)30 detected total poly(A)+ RNA. Nuclear DNA was visualized by DAPI staining. *, cell undergoing apoptosis. (C) Poly(A)+ RNA distribution in untreated HeLa cells shows distribution similar to AP–hGle1-scrSD–treated cells. (D) AP–hGle1-SD impairs nuclear export of DHFR mRNA. Cells treated with AP–peptides were processed for in situ hybridization using a digoxigenin-labeled DNA probe against DHFR mRNA. Bar, 10 μm. (B–D) Respective hybridization signals represent equivalent exposure times. (E) Semiquantitative analysis of intracellular DHFR mRNA distribution in subcellular fractions of AP–peptide-treated cells. After treatment with peptides, total RNA from nuclear and cytoplasmic fractions was isolated and reverse transcribed with oligo (dT)18, and serial dilutions were made. Subsaturating PCR-based amplifications with DHFR-specific primers were performed (see Materials and methods), and products were separated on agarose gels and stained with ethidium bromide.
Figure 5.
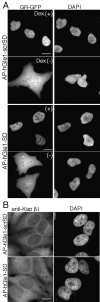
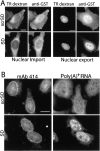
Nuclear protein import and export are not perturbed in HeLa cells treated with AP–peptides. (A) HeLa cells transfected with a GR–GFP expression vector were treated with 5 μM of AP–peptide. Dexamethasone (Dex+) or an equivalent volume of ethanol (Dex−) was added in the last 30 min before fixation and DAPI staining. Dexamethasone addition specifically induces nuclear import of the cytoplasmic GR–GFP pool in the presence of either peptide. (B) Karyopherin/importin β1 (Kapβ1) intracellular localization in AP–hGle1-SD– and control peptide (scrSD)–treated cells is not perturbed. Cells were treated with peptides as described above, fixed, and processed for IIF using anti-Kapβ1 antibodies. Bars, 10 μm.
Figure 6.
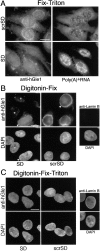
AP–hGle1-SD peptide results in mislocalization of endogenous hGle1 localization when poly(A) + RNA export is inhibited. HeLa cells were incubated with AP–hGle1-SD (SD) or control peptide (scrSD), processed for IIF using affinity-purified anti-hGle1 antibodies (A–C), and in situ hybridized with oligo (dT)30 (A) or stained with DAPI (B and C). Hybridized probe and bound hGle1 antibodies were simultaneously detected using rhodamine-labeled anti–Dig Fab antibodies and FITC-labeled anti–rabbit antibodies, respectively. Images showing in situ hybridization results reflect equivalent exposure times. (A) Fix-Triton permeabilization detects total hGle1 pool and shows changes in anti-hGle1 localization in AP–hGle1-SD–treated cells. (B) Digitonin-Fix permeabilization detects only the cytoplasmically accessible pool. AP–hGle1-SD results in decreased levels of cytoplasmically accessible hGle1 (asterisk) compared with unaffected cells (arrowheads, at passage >150; see Materials and methods) or cells treated with control peptide. The absence of lamin B staining using anti–lamin B antibodies (far right) confirms NE integrity under these conditions. (C) Detection of nuclear hGle1 was accomplished by combined Digitonin-Fix-Triton permeabilization. Lamin B staining confirms access of antibodies to intranuclear proteins. Bars, 10 μm.
Figure 7.
The AP–hGle1-SD peptide inhibits the shuttling of GST–hGle1. (A) HeLa cells were pretreated with AP–peptides (3 h) and microinjected with GST–hGle1A in the cytoplasm (left) or nucleus (right) of the cells. After recovery (2 h), the intracellular distribution of GST–hGle1A was analyzed by IIF using anti-GST antibodies. Compared with cells treated with the scrSD control peptide, less GST–hGle1 is detected outside the microinjection site in the majority of cells analyzed. (B) NPC/NE association of nucleoporins recognized by mAb414 is not perturbed by AP–hGle1-SD treatment. HeLa cells incubated with AP–hGle1-SD (SD) or control peptide (scrSD) were processed for in situ hybridization with oligo(dT)30 and coincident IIF with mAb414. The mAb414 staining is similar in AP–hGle1-SD and scrSD cells despite the poly(A)+ RNA export defect in the AP–hGle1-SD cells. Bars, 10 μm.
Figure 8.
Comparison of the hGle1 SD with nucleocytoplasmic shuttling sequences. The amino acid sequence of the hGle1 SD does not share significant similarities with other nucleocytoplasmic transport signals (Michael et al., 1995, 1997; Fan and Steitz, 1998). There are limited similarities with the hnRNP A1 M9 transport signal (boxed), but the hGle1 SD lacks the critical GPM triplet (asterisks) required for efficient transport activity of M9 (Bogerd et al., 1999).
Similar articles
- The mRNA export factor human Gle1 interacts with the nuclear pore complex protein Nup155.
Rayala HJ, Kendirgi F, Barry DM, Majerus PW, Wente SR. Rayala HJ, et al. Mol Cell Proteomics. 2004 Feb;3(2):145-55. doi: 10.1074/mcp.M300106-MCP200. Epub 2003 Nov 25. Mol Cell Proteomics. 2004. PMID: 14645504 - Interaction between the shuttling mRNA export factor Gle1 and the nucleoporin hCG1: a conserved mechanism in the export of Hsp70 mRNA.
Kendirgi F, Rexer DJ, Alcázar-Román AR, Onishko HM, Wente SR. Kendirgi F, et al. Mol Biol Cell. 2005 Sep;16(9):4304-15. doi: 10.1091/mbc.e04-11-0998. Epub 2005 Jul 6. Mol Biol Cell. 2005. PMID: 16000379 Free PMC article. - An amyotrophic lateral sclerosis-linked mutation in GLE1 alters the cellular pool of human Gle1 functional isoforms.
Aditi, Glass L, Dawson TR, Wente SR. Aditi, et al. Adv Biol Regul. 2016 Sep;62:25-36. doi: 10.1016/j.jbior.2015.11.001. Epub 2015 Nov 11. Adv Biol Regul. 2016. PMID: 26776475 Free PMC article. - Transport of messenger RNA from the nucleus to the cytoplasm.
Cole CN, Scarcelli JJ. Cole CN, et al. Curr Opin Cell Biol. 2006 Jun;18(3):299-306. doi: 10.1016/j.ceb.2006.04.006. Epub 2006 May 8. Curr Opin Cell Biol. 2006. PMID: 16682182 Review. - Nucleocytoplasmic shuttling signals: two for the price of one.
Michael WM. Michael WM. Trends Cell Biol. 2000 Feb;10(2):46-50. doi: 10.1016/s0962-8924(99)01695-5. Trends Cell Biol. 2000. PMID: 10652514 Review.
Cited by
- A mitotic nuclear envelope tether for Gle1 also impacts nuclear and nucleolar architecture.
Chemudupati M, Osmani AH, Osmani SA. Chemudupati M, et al. Mol Biol Cell. 2016 Sep 14;27(23):3757-70. doi: 10.1091/mbc.E16-07-0544. Online ahead of print. Mol Biol Cell. 2016. PMID: 27630260 Free PMC article. - Nucleocytoplasmic shuttling of Gle1 impacts DDX1 at transcription termination sites.
Sharma M, Wente SR. Sharma M, et al. Mol Biol Cell. 2020 Oct 1;31(21):2398-2408. doi: 10.1091/mbc.E20-03-0215. Epub 2020 Aug 5. Mol Biol Cell. 2020. PMID: 32755435 Free PMC article. - Nup42 and IP6 coordinate Gle1 stimulation of Dbp5/DDX19B for mRNA export in yeast and human cells.
Adams RL, Mason AC, Glass L, Aditi, Wente SR. Adams RL, et al. Traffic. 2017 Dec;18(12):776-790. doi: 10.1111/tra.12526. Epub 2017 Oct 16. Traffic. 2017. PMID: 28869701 Free PMC article. - Cytoplasmic hGle1A regulates stress granules by modulation of translation.
Aditi, Folkmann AW, Wente SR. Aditi, et al. Mol Biol Cell. 2015 Apr 15;26(8):1476-90. doi: 10.1091/mbc.E14-11-1523. Epub 2015 Feb 18. Mol Biol Cell. 2015. PMID: 25694449 Free PMC article. - A Kaposi's sarcoma virus RNA element that increases the nuclear abundance of intronless transcripts.
Conrad NK, Steitz JA. Conrad NK, et al. EMBO J. 2005 May 18;24(10):1831-41. doi: 10.1038/sj.emboj.7600662. Epub 2005 Apr 28. EMBO J. 2005. PMID: 15861127 Free PMC article.
References
- Bachi, A., I.C. Braun, J.P. Rodrigues, N. Pante, K. Ribbeck, C. Von Kobbe, U. Kutay, M. Wilm, D. Gorlich, M. Carmo-Fonseca, and E. Izaurralde. 2000. The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA. 6:136–158. - PMC - PubMed
- Bogerd, H.P., R.E. Benson, R. Truant, A. Herold, M. Phingbodhipakkiya, and B.R. Cullen. 1999. Definition of a consensus transportin-specific nucleocytoplasmic transport signal. J. Biol. Chem. 274:9771–9777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM008367/GM/NIGMS NIH HHS/United States
- R01 GM051219/GM/NIGMS NIH HHS/United States
- GM-197190/GM/NIGMS NIH HHS/United States
- GM-59975/GM/NIGMS NIH HHS/United States
- GM-51219/GM/NIGMS NIH HHS/United States
- R37 GM051219/GM/NIGMS NIH HHS/United States
- T32 GM08367-13/GM/NIGMS NIH HHS/United States
- R01 GM059975/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases