Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol - PubMed (original) (raw)
Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol
David G Breckenridge et al. J Cell Biol. 2003.
Abstract
Stimulation of cell surface death receptors activates caspase-8, which targets a limited number of substrates including BAP31, an integral membrane protein of the endoplasmic reticulum (ER). Recently, we reported that a caspase-resistant BAP31 mutant inhibited several features of Fas-induced apoptosis, including the release of cytochrome c (cyt.c) from mitochondria (Nguyen, M., D.G. Breckenridge, A. Ducret, and G.C. Shore. 2000. Mol. Cell. Biol. 20:6731-6740), implicating ER-mitochondria crosstalk in this pathway. Here, we report that the p20 caspase cleavage fragment of BAP31 can direct pro-apoptotic signals between the ER and mitochondria. Adenoviral expression of p20 caused an early release of Ca2+ from the ER, concomitant uptake of Ca2+ into mitochondria, and mitochondrial recruitment of Drp1, a dynamin-related protein that mediates scission of the outer mitochondrial membrane, resulting in dramatic fragmentation and fission of the mitochondrial network. Inhibition of Drp1 or ER-mitochondrial Ca2+ signaling prevented p20-induced fission of mitochondria. p20 strongly sensitized mitochondria to caspase-8-induced cyt.c release, whereas prolonged expression of p20 on its own ultimately induced caspase activation and apoptosis through the mitochondrial apoptosome stress pathway. Therefore, caspase-8 cleavage of BAP31 at the ER stimulates Ca2+-dependent mitochondrial fission, enhancing the release of cyt.c in response to this initiator caspase.
Figures
Figure 1.
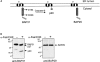
BAP31, but not BAP29, is cleaved during Fas-mediated apoptosis. (A) Schematic representation of human BAP31, the p20 caspase cleavage product, and BAP29 in the ER membrane. Both BAP31 and BAP29 contain three transmembrane domains, a cytosolic tail containing a coiled coil domain (boxed region), and terminate with a canonical KKXX ER retrieval sequence. The caspase-8 recognition sites in BAP31 are shown. (B) KB cells were untreated or stimulated with 500 ng/ml anti-Fas activating antibody (CH11) and 10 μg/ml cycloheximide (CHX) for 7 h, and cell lysates were analyzed by SDS-PAGE and immunoblotting with anti-BAP31 (left) or anti-BAP29 (right) pAbs. The positions of BAP31, its p27 and p20 cleavage products, and BAP29 are indicated. (C) Adenoviral-expressed p20-HA (Adp20) localizes to the ER. H1299 cells were infected with Adp20 for 20 h, then fixed and double stained with anti-HA and anti-calreticulin antibodies or anti-HA and anti-TOM20 antibodies.
Figure 1.
BAP31, but not BAP29, is cleaved during Fas-mediated apoptosis. (A) Schematic representation of human BAP31, the p20 caspase cleavage product, and BAP29 in the ER membrane. Both BAP31 and BAP29 contain three transmembrane domains, a cytosolic tail containing a coiled coil domain (boxed region), and terminate with a canonical KKXX ER retrieval sequence. The caspase-8 recognition sites in BAP31 are shown. (B) KB cells were untreated or stimulated with 500 ng/ml anti-Fas activating antibody (CH11) and 10 μg/ml cycloheximide (CHX) for 7 h, and cell lysates were analyzed by SDS-PAGE and immunoblotting with anti-BAP31 (left) or anti-BAP29 (right) pAbs. The positions of BAP31, its p27 and p20 cleavage products, and BAP29 are indicated. (C) Adenoviral-expressed p20-HA (Adp20) localizes to the ER. H1299 cells were infected with Adp20 for 20 h, then fixed and double stained with anti-HA and anti-calreticulin antibodies or anti-HA and anti-TOM20 antibodies.
Figure 2.
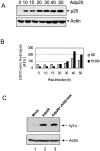
Prolonged expression of p20 induces mitochondrial apoptosis. (A) Expression of p20 in KB cells. Cells were infected with Adp20 and cell lysates were collected and analyzed by immunoblotting at the times indicated post-infection. (B) KB and H1299 cells were infected with Adp20, and effector caspase (DEVDase) activity was measured at the indicated times post-infection by the ability of cell lysates to hydrolyze the fluorogenic caspase substrate DEVD-amc. Shown is a representative experiment. (C) KB cells were mock infected or infected with Adp20 for 35–40 h in the absence or presence of 50 μM zVAD-fmk, and equivalent amounts of post-mitochondrial supernatants were analyzed for the presence of cyt.c by SDS-PAGE and immunoblotting. The membrane was reprobed with anti-actin antibody to confirm equal loading. (D) Parental KB cells, or KB cells stably overexpressing BCL-2 or BCL-xL, were mock infected or infected with Adp20 in the absence or presence of 50 μM zVAD-fmk, and at 45 h post infection, cell death was assessed by trypan blue staining. Shown is mean ± SD of three independent experiments. (E) Wt, _Bap31_-null, and _Bap29,31_-null mouse ES cells were treated and analyzed as in D.
Figure 2.
Prolonged expression of p20 induces mitochondrial apoptosis. (A) Expression of p20 in KB cells. Cells were infected with Adp20 and cell lysates were collected and analyzed by immunoblotting at the times indicated post-infection. (B) KB and H1299 cells were infected with Adp20, and effector caspase (DEVDase) activity was measured at the indicated times post-infection by the ability of cell lysates to hydrolyze the fluorogenic caspase substrate DEVD-amc. Shown is a representative experiment. (C) KB cells were mock infected or infected with Adp20 for 35–40 h in the absence or presence of 50 μM zVAD-fmk, and equivalent amounts of post-mitochondrial supernatants were analyzed for the presence of cyt.c by SDS-PAGE and immunoblotting. The membrane was reprobed with anti-actin antibody to confirm equal loading. (D) Parental KB cells, or KB cells stably overexpressing BCL-2 or BCL-xL, were mock infected or infected with Adp20 in the absence or presence of 50 μM zVAD-fmk, and at 45 h post infection, cell death was assessed by trypan blue staining. Shown is mean ± SD of three independent experiments. (E) Wt, _Bap31_-null, and _Bap29,31_-null mouse ES cells were treated and analyzed as in D.
Figure 2.
Prolonged expression of p20 induces mitochondrial apoptosis. (A) Expression of p20 in KB cells. Cells were infected with Adp20 and cell lysates were collected and analyzed by immunoblotting at the times indicated post-infection. (B) KB and H1299 cells were infected with Adp20, and effector caspase (DEVDase) activity was measured at the indicated times post-infection by the ability of cell lysates to hydrolyze the fluorogenic caspase substrate DEVD-amc. Shown is a representative experiment. (C) KB cells were mock infected or infected with Adp20 for 35–40 h in the absence or presence of 50 μM zVAD-fmk, and equivalent amounts of post-mitochondrial supernatants were analyzed for the presence of cyt.c by SDS-PAGE and immunoblotting. The membrane was reprobed with anti-actin antibody to confirm equal loading. (D) Parental KB cells, or KB cells stably overexpressing BCL-2 or BCL-xL, were mock infected or infected with Adp20 in the absence or presence of 50 μM zVAD-fmk, and at 45 h post infection, cell death was assessed by trypan blue staining. Shown is mean ± SD of three independent experiments. (E) Wt, _Bap31_-null, and _Bap29,31_-null mouse ES cells were treated and analyzed as in D.
Figure 3.
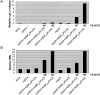
p20 sensitizes mitochondria to caspase-8–induced cyt.c release. H1299 cells were mock infected, or co-infected with AdRTA (control) and AdMFpk3FLICE or with Adp20 and AdMFpk3FLICE. 16 h post-infection, FK1012Z or vehicle alone (DMSO; Wang et al., 2003) were added for 45 or 90 min, and the amount of cyt.c in the post-mitochondrial supernatant and tBID in the mitochondrial fraction were assessed by SDS-PAGE and Western blot. The intensity of the cyt.c (A) and tBID (B) signals, relative to loading controls, was determined using ImageQuantTM software (Amersham Biosciences) and is expressed in arbitrary units. Shown is a representative of three independent experiments.
Figure 4.
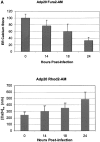
p20-induced mitochondrial fragmentation is mediated by an ER-mitochondria Ca2 + signal. (A) Top, p20 induces a time dependent release of Ca2+ from the ER. H1299 cells were infected with Adp20 in the presence of 50 μM zVAD-fmk and at the indicated times post-infection, cells were loaded with Fura2-AM in Ca2+-free buffer and ER calcium stores were measured as the sudden difference in Fura2 fluorescence recorded after the addition of TG (see Materials and methods). Shown is the mean and SD of five independent experiments. Bottom, elevated [Ca2+]m after Adp20 infection. HeLa cells were treated as in A, except cells were loaded with Rhod2-AM and the [Ca2+]m was estimated as described in the Materials and methods. (B) p20 induces dramatic fragmentation of mitochondria, which is inhibited by predepletion of ER Ca2+ stores with TG. H1299 cells were infected with 50 μM Adp20 + zVAD-fmk in the absence or presence of 50 nm TG for 24 h, and mitochondria were visualized by anti-cyt.c staining. Representative images are shown. (C) Reducing ER Ca2+ stores, chelating cytosolic Ca2+, or preventing mitochondrial Ca2+ uptake inhibits p20-induced fragmentation of mitochondria. As in B, but H1229 cells, or H1299 cells pretreated with 50 nm TG, 2 μM BAPTA-AM, or 20 μM Ru360, or H1299 b5-BCL-2 cells were infected with Adp20 + zVAD-fmk for 24 h and the number of cells showing signs of mitochondrial fragmentation was quantified. Shown is the mean ± SD of five independent experiments.
Figure 4.
p20-induced mitochondrial fragmentation is mediated by an ER-mitochondria Ca2 + signal. (A) Top, p20 induces a time dependent release of Ca2+ from the ER. H1299 cells were infected with Adp20 in the presence of 50 μM zVAD-fmk and at the indicated times post-infection, cells were loaded with Fura2-AM in Ca2+-free buffer and ER calcium stores were measured as the sudden difference in Fura2 fluorescence recorded after the addition of TG (see Materials and methods). Shown is the mean and SD of five independent experiments. Bottom, elevated [Ca2+]m after Adp20 infection. HeLa cells were treated as in A, except cells were loaded with Rhod2-AM and the [Ca2+]m was estimated as described in the Materials and methods. (B) p20 induces dramatic fragmentation of mitochondria, which is inhibited by predepletion of ER Ca2+ stores with TG. H1299 cells were infected with 50 μM Adp20 + zVAD-fmk in the absence or presence of 50 nm TG for 24 h, and mitochondria were visualized by anti-cyt.c staining. Representative images are shown. (C) Reducing ER Ca2+ stores, chelating cytosolic Ca2+, or preventing mitochondrial Ca2+ uptake inhibits p20-induced fragmentation of mitochondria. As in B, but H1229 cells, or H1299 cells pretreated with 50 nm TG, 2 μM BAPTA-AM, or 20 μM Ru360, or H1299 b5-BCL-2 cells were infected with Adp20 + zVAD-fmk for 24 h and the number of cells showing signs of mitochondrial fragmentation was quantified. Shown is the mean ± SD of five independent experiments.
Figure 4.
p20-induced mitochondrial fragmentation is mediated by an ER-mitochondria Ca2 + signal. (A) Top, p20 induces a time dependent release of Ca2+ from the ER. H1299 cells were infected with Adp20 in the presence of 50 μM zVAD-fmk and at the indicated times post-infection, cells were loaded with Fura2-AM in Ca2+-free buffer and ER calcium stores were measured as the sudden difference in Fura2 fluorescence recorded after the addition of TG (see Materials and methods). Shown is the mean and SD of five independent experiments. Bottom, elevated [Ca2+]m after Adp20 infection. HeLa cells were treated as in A, except cells were loaded with Rhod2-AM and the [Ca2+]m was estimated as described in the Materials and methods. (B) p20 induces dramatic fragmentation of mitochondria, which is inhibited by predepletion of ER Ca2+ stores with TG. H1299 cells were infected with 50 μM Adp20 + zVAD-fmk in the absence or presence of 50 nm TG for 24 h, and mitochondria were visualized by anti-cyt.c staining. Representative images are shown. (C) Reducing ER Ca2+ stores, chelating cytosolic Ca2+, or preventing mitochondrial Ca2+ uptake inhibits p20-induced fragmentation of mitochondria. As in B, but H1229 cells, or H1299 cells pretreated with 50 nm TG, 2 μM BAPTA-AM, or 20 μM Ru360, or H1299 b5-BCL-2 cells were infected with Adp20 + zVAD-fmk for 24 h and the number of cells showing signs of mitochondrial fragmentation was quantified. Shown is the mean ± SD of five independent experiments.
Figure 5.
p20 induces fragmentation of the mitochondrial network as an early event. (A) Mitochondrial restructuring and fragmentation occur in the absence of cell shrinkage. Rat1 fibroblasts were infected with Adp20 in the presence of 50 μM zVAD-fmk (to prevent caspase activation and cell detachment) for 20 h, fixed, and double stained with anti-tubulin and anti-TOM20 antibodies. (B) Mitochondrial fragmentation occurs before activation of BAX and cyt.c release. As in A, except cells were infected for 25 h and double stained with anti-cyt.c antibody (arrows, lower left) and the active conformation-specific anti-BAX-NT antibody (aa 1–21, Upstate Biotechnology; arrows, lower right).
Figure 6.
Drp1 mediates p20-induced mitochondrial fission. (A) Recruitment of endogenous Drp1 to mitochondria. HeLa cells were mock infected (top) or infected with Adp20 (bottom) in the presence of zVAD-fmk, and 17 h post-infection, cells were fixed, double stained with anti-Drp1 (green) and anti-TOM20 (red) antibodies, and imaged by confocal immunofluorescence microscopy. Enlargement of the merged overlay revealed that clusters of Drp1 relocate along mitochondrial filaments before the onset of fission. (B) H1299 cells, H1299 cells treated with TG, or H1299 b5-BCL-2 cells were infected with Adp20+zVAD for 18 h and the mitochondrial fraction was isolated and analyzed for the presence of Drp1 by SDS-PAGE and immunoblotting. The blot was reprobed with anti-TOM20 antibody to demonstrate equal protein loading.
Figure 7.
Expression of a Drp1K38E dominant-negative mutant inhibits p20 induced disruption of the mitochondrial network. (A) Rat1 fibroblasts were transiently transfected with CFP-Drp1 or CFP- Drp1K38E, then either mock infected or infected with Adp20 in the presence of zVAD-fmk. 24 h post-infection cells were fixed, stained with anti-TOM20, and analyzed by fluorescence microscopy. Cells expressing CFP-Drp1 or CFP- Drp1K38E were identified under the cyan filter and are indicated with an arrow. (B) CFP- Drp1K38E inhibits cyt.c release. H1299 cells were treated as in B for 36 h, and immunofluorescence microscopy was used to assess the distribution of cyt.c in cells positive for CFP fluorescence. Shown is the mean ± SD of four independent experiments. (C) H1299 cells were transiently cotransfected with the indicated constructs and 36 h post-transfection cell lysates were collected and processed for DEVDase activity, shown is the mean ± SD of three independent experiments.
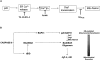
Figure 8.
A proposed mechanism of p20-induced mitochondrial fission. (A) p20 triggers a specific Ca2+ signal from the ER that is decoded by mitochondria. Mitochondria, in turn, recruit Drp1, which initiates organelle fission. Lowering ER Ca2+ stores by pretreatment with TG or expression of b5-BCL-2, chelating the Ca2+ released to the cytosol with BAPTA, blocking mitochondrial uptake of Ca2+ with Ru360, or inhibition of Drp1 by expression of Drp1K38E all prevent p20-induced mitochondrial fission. (B) A model depicting how in intact cells, cleavage of BAP31 at the ER sensitizes mitochondria to caspase-8–driven cyt.c release. Stimulation of Fas leads to caspase-8–dependent processing of BAP31 and BID, generating p20 and tBID. tBID translocates to mitochondria, where it induces the oligomerization of BAX/BAK into pores in the OMM. Simultaneously, p20 triggers ER Ca2+ release, causing Drp-1 translocation to mitochondria and subsequent organelle fission, enhancing the release of cyt.c to the cytosol.
Similar articles
- Caspase-resistant BAP31 inhibits fas-mediated apoptotic membrane fragmentation and release of cytochrome c from mitochondria.
Nguyen M, Breckenridge DG, Ducret A, Shore GC. Nguyen M, et al. Mol Cell Biol. 2000 Sep;20(18):6731-40. doi: 10.1128/MCB.20.18.6731-6740.2000. Mol Cell Biol. 2000. PMID: 10958671 Free PMC article. - Uncleaved BAP31 in association with A4 protein at the endoplasmic reticulum is an inhibitor of Fas-initiated release of cytochrome c from mitochondria.
Wang B, Nguyen M, Breckenridge DG, Stojanovic M, Clemons PA, Kuppig S, Shore GC. Wang B, et al. J Biol Chem. 2003 Apr 18;278(16):14461-8. doi: 10.1074/jbc.M209684200. Epub 2003 Jan 15. J Biol Chem. 2003. PMID: 12529377 - Rapid cytochrome c release, activation of caspases 3, 6, 7 and 8 followed by Bap31 cleavage in HeLa cells treated with photodynamic therapy.
Granville DJ, Carthy CM, Jiang H, Shore GC, McManus BM, Hunt DW. Granville DJ, et al. FEBS Lett. 1998 Oct 16;437(1-2):5-10. doi: 10.1016/s0014-5793(98)01193-4. FEBS Lett. 1998. PMID: 9804161 - The ER-mitochondria interface: the social network of cell death.
Grimm S. Grimm S. Biochim Biophys Acta. 2012 Feb;1823(2):327-34. doi: 10.1016/j.bbamcr.2011.11.018. Epub 2011 Dec 13. Biochim Biophys Acta. 2012. PMID: 22182703 Review. - Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra.
Ferrer I, Planas AM. Ferrer I, et al. J Neuropathol Exp Neurol. 2003 Apr;62(4):329-39. doi: 10.1093/jnen/62.4.329. J Neuropathol Exp Neurol. 2003. PMID: 12722825 Review.
Cited by
- Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca²⁺ and reactive oxygen species signaling.
Eisner V, Csordás G, Hajnóczky G. Eisner V, et al. J Cell Sci. 2013 Jul 15;126(Pt 14):2965-78. doi: 10.1242/jcs.093609. Epub 2013 Jul 10. J Cell Sci. 2013. PMID: 23843617 Free PMC article. Review. - Mitochondrial function in vascular endothelial cell in diabetes.
Pangare M, Makino A. Pangare M, et al. J Smooth Muscle Res. 2012;48(1):1-26. doi: 10.1540/jsmr.48.1. J Smooth Muscle Res. 2012. PMID: 22504486 Free PMC article. Review. - Chemical Modulation of Mitochondria-Endoplasmic Reticulum Contact Sites.
Magalhães Rebelo AP, Dal Bello F, Knedlik T, Kaar N, Volpin F, Shin SH, Giacomello M. Magalhães Rebelo AP, et al. Cells. 2020 Jul 7;9(7):1637. doi: 10.3390/cells9071637. Cells. 2020. PMID: 32646031 Free PMC article. Review. - Host Mitochondrial Requirements of Cytomegalovirus Replication.
Monk CH, Zwezdaryk KJ. Monk CH, et al. Curr Clin Microbiol Rep. 2020 Dec;7(4):115-123. doi: 10.1007/s40588-020-00153-5. Epub 2020 Sep 30. Curr Clin Microbiol Rep. 2020. PMID: 33816061 Free PMC article. - The E3 Ubiquitin Ligase SYVN1 Plays an Antiapoptotic Role in Polycystic Ovary Syndrome by Regulating Mitochondrial Fission.
Sun L, Ye H, Tian H, Xu L, Cai J, Zhang C, Wang R, Yang H, Zhao S, Zhang J, Gao S. Sun L, et al. Oxid Med Cell Longev. 2022 Sep 19;2022:3639302. doi: 10.1155/2022/3639302. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36193086 Free PMC article.
References
- Bereiter-Hahn, J., and M. Voth. 1994. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc. Res. Tech. 27:198–219. - PubMed
- Berridge, M.J., P. Lipp, and M.D. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous