Bone marrow transplantation reveals an essential synergy between neuronal and hemopoietic cell neurokinin production in pulmonary inflammation - PubMed (original) (raw)
Bone marrow transplantation reveals an essential synergy between neuronal and hemopoietic cell neurokinin production in pulmonary inflammation
Mara Chavolla-Calderón et al. J Clin Invest. 2003 Apr.
Abstract
Neurogenic inflammation is believed to originate with the antidromic release of substance P, and of other neurokinins encoded by the preprotachykinin A (PPT-A) gene, from unmyelinated nerve fibers (C-fibers) following noxious stimuli. Consistent with this concept, we show here that selective sensory-fiber denervation with capsaicin and targeted deletion of the PPT-A gene protect murine lungs against both immune complex-mediated and stretch-mediated injuries. Reconstitution of PPT-A gene-deleted mice with WT bone marrow does not abrogate this protection, demonstrating a critical role for PPT-A gene expression by sensory neurons in pulmonary inflammation. Surprisingly, reconstitution of WT mice with PPT-A gene-deficient bone marrow also confers protection against pulmonary injury, revealing that PPT-A gene expression in hemopoietic cells has a previously unanticipated essential role in tissue injury. Taken together, these findings demonstrate a critical synergy between capsaicin-sensitive sensory fibers and hemopoietic cells in neurokinin-mediated inflammation and suggest that such synergy may be the basis for a stereotypical mechanism of response to injury in the respiratory tract.
Figures
Figure 1
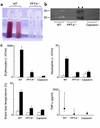
Attenuation of immune complex–mediated injury by PPT-A gene deletion and capsaicin-selective sensory denervation. (a) Bronchoalveolar lavage fluid obtained after intravenous injection of chicken ovalbumin and intratracheal instillation of a polyclonal antibody against ovalbumin shows alveolar hemorrhage in WT mice, but not in mice with targeted deletion of the PPT-A gene (_PPT-A_–/–). (b) Casein zymogram demonstrates increased bronchoalveolar lavage fluid protease activity in WT compared with _PPT-A_–/– and capsaicin-denervated mice. The proteolytic bands visible in the illustration have the electrophoretic mobility profiles of matrilysin and macrophage metalloelastase. Black arrowheads indicate molecular weight markers. (c) Erythrocyte and neutrophil counts in the bronchoalveolar lavage fluid, Evans blue lavage/serum concentration ratios, and TNF-α levels in the bronchoalveolar lavage fluid demonstrate protection against immune complex injury by PPT-A gene deletion and capsaicin denervation (P < 0.0001, except for TNF-α levels where P = 0.01). Cell counts and Evans blue ratios (black bars; n = 18 WT, 11 _PPT-A_–/–, and 19 capsaicin) are compared with those of control mice injected intravenously with ovalbumin and intratracheally with normal saline (white bars; n = 8 WT, 2 _PPT-A_–/–, and 12 capsaicin). TNF-α levels were measured in fewer mice (n = 10 WT, 7 _PPT-A_–/–, and 11 capsaicin). Values are shown as mean ± SE, except for TNF-α levels, which are shown as median and 25th to 75th percentile span.
Figure 2
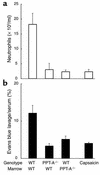
Protection conferred against stretch-mediated injury by PPT-A gene deletion. (a) H&E-stained sections (×20) obtained 24 hours after a 4-hour period of mechanical ventilation with a tidal volume of 20 ml/kg demonstrate edema and inflammatory infiltration of the pulmonary interstitium in WT mouse but not in PPT-A gene–deleted (_PPT-A_–/–) mouse. (b) Microphotograph (×40) of bronchoalveolar lavage fluid after similar treatment shows exudation of macrophages and neutrophils in WT mouse, but only small numbers of macrophages in _PPT-A_–/– mouse. (c) Cell counts in the bronchoalveolar lavage fluid (n = 10 for each combination of variables)show increased numbers of neutrophils (gray bars), macrophages (white bars), and erythrocytes (black bars) in WT but not in _PPT-A_–/– mice 24 hours after ventilation (vent + 24 h) with high tidal volumes (High V) (P < 0.0001). (d) Evans blue lavage/serum ratios (n = 10)demonstrate increased alveolar-capillary permeability in WT but not in _PPT-A_–/– mice 24 hours after High V (P < 0.002). (e) TNF-α levels (black bars; n = 5) were higher in WT than in _PPT-A_–/– mice after ventilation with High V, both at the end of the 4-hour ventilation period (vent + 0 h; P = 0.02) and 24 hours later (P < 0.0001). MIP-1α levels (white bars; n = 5) were higher in WT than in _PPT-A_–/– mice after High V, but only after 24 hours (P < 0.0001). Values are shown as mean ± SE.
Figure 3
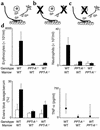
Effects of bone marrow reconstitution with WT cells in WT and PPT-A gene–deleted (_PPT-A_–/–) mice and with _PPT-A_–/– cells in WT mice on immune complex–mediated lung injury. (a) WT mice reconstituted with WT bone marrow after conditioning irradiation. (b) _PPT-A_–/– mice (shown by crossing of cells affected by the gene deletion) reconstituted with WT bone marrow, restoring the ability of their hemopoietic cells to produce substance P (SP) and other PPT-A gene–encoded neurokinins. (c) WT mice reconstituted with _PPT-A_–/– bone marrow, eliminating the ability of their hemopoietic cells to produce PPT-A gene–encoded neurokinins. (d) WT mice reconstituted with WT bone marrow (n = 12) developed intense inflammation after immune complex formation. Reconstitution of _PPT-A_–/– mice with WT bone marrow did not reestablish the inflammatory response (n = 15; P < 0.0001). Reconstitution of WT mice with _PPT-A_–/– bone marrow protected against this response (n = 15; P < 0.0001). Cell counts and Evans blue ratios are shown in comparison with control mice injected intravenously with ovalbumin and intratracheally with normal saline (white bars; n = 4 WT bone marrow to WT, 6 _PPT-A_–/– bone marrow to WT, and 3 WT bone marrow to _PPT-A_–/– mice). TNF-α levels were determined in fewer mice (n = 7, 10, and 12, respectively). Values are shown as mean ± SE, except for TNF-α levels, which are shown as median and 25th to 75th percentile span.
Figure 4
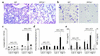
(a–c) Immunostaining of lung tissue for substance P after immune complex–mediated injury. Substance P immunoreactivity (IR, red arrows) was detected only in macrophages (but not other inflammatory cells) of WT mice reconstituted with WT bone marrow (a) and PPT-A gene–deleted mice reconstituted with WT bone marrow (b). No substance P immunoreactivity was present in macrophages (green arrows) or other cells in PPT-A gene–deleted mice (c). (d) Decreased substance P immunoreactivity (IR), by ELISA, in the bronchoalveolar fluid of WT, PPT-A gene–deleted (_PPT-A_–/–) mice reconstituted with WT bone marrow, WT mice reconstituted with _PPT-_A–/– bone marrow, and capsaicin-pretreated mice, after an immune complex–mediated lung injury (P = 0.03). Comparisons are relative to WT mice reconstituted with WT bone marrow, which serve as a WT control for the effects of conditioning irradiation. Values are shown as mean ± SE (n = 5 for all groups).
Figure 5
Protection conferred by reconstitution of WT mice with PPT-A gene–deleted (_PPT-A_–/–) bone marrow and by capsaicin-selective denervation against inflammation (neutrophil counts in bronchoalveolar fluid; P = 0.0005) and increased alveolar-capillary permeability (Evans blue lavage fluid/serum ratio; P = 0.0014) 24 hours after a 4-hour period of high-volume ventilation. These results demonstrate, in a manner similar to that shown for immune complex–mediated inflammation (see Figure 3), that PPT-A gene expression by hemopoietic cells is critical for the progression of stretch-mediated inflammation. Values are shown as mean ± SE (n = 5 for WT-to-WT reconstitution and 4 for all other groups).
Similar articles
- Role of preprotachykinin-A gene products on multiple organ injury in LPS-induced endotoxemia.
Ng SW, Zhang H, Hegde A, Bhatia M. Ng SW, et al. J Leukoc Biol. 2008 Feb;83(2):288-95. doi: 10.1189/jlb.0807575. Epub 2007 Nov 12. J Leukoc Biol. 2008. PMID: 17998302 - Early protection from burn-induced acute lung injury by deletion of preprotachykinin-A gene.
Sio SW, Moochhala S, Lu J, Bhatia M. Sio SW, et al. Am J Respir Crit Care Med. 2010 Jan 1;181(1):36-46. doi: 10.1164/rccm.200907-1073OC. Epub 2009 Oct 1. Am J Respir Crit Care Med. 2010. PMID: 19797759 - A role for tachykinins in female mouse and rat reproductive function.
Pintado CO, Pinto FM, Pennefather JN, Hidalgo A, Baamonde A, Sanchez T, Candenas ML. Pintado CO, et al. Biol Reprod. 2003 Sep;69(3):940-6. doi: 10.1095/biolreprod.103.017111. Epub 2003 May 28. Biol Reprod. 2003. PMID: 12773411 - Tachykinin-mediated modulation of the immune response.
Bost KL. Bost KL. Front Biosci. 2004 Sep 1;9:3331-2. doi: 10.2741/1484. Front Biosci. 2004. PMID: 15358592 Review. - Hematopoietic regulation mediated by interactions among the neurokinins and cytokines.
Rameshwar P, Poddar A, Gascón P. Rameshwar P, et al. Leuk Lymphoma. 1997 Dec;28(1-2):1-10. doi: 10.3109/10428199709058325. Leuk Lymphoma. 1997. PMID: 9498698 Review.
Cited by
- Tachykinins and Neurokinin Receptors in Bone Marrow Functions: Neural-Hematopoietic Link.
Klassert TE, Patel SA, Rameshwar P. Klassert TE, et al. J Receptor Ligand Channel Res. 2010 Apr 1;2010(3):51-61. doi: 10.2147/jrlcr.s6509. J Receptor Ligand Channel Res. 2010. PMID: 20593004 Free PMC article. - Respiratory mechanics in brain injury: A review.
Koutsoukou A, Katsiari M, Orfanos SE, Kotanidou A, Daganou M, Kyriakopoulou M, Koulouris NG, Rovina N. Koutsoukou A, et al. World J Crit Care Med. 2016 Feb 4;5(1):65-73. doi: 10.5492/wjccm.v5.i1.65. eCollection 2016 Feb 4. World J Crit Care Med. 2016. PMID: 26855895 Free PMC article. Review. - Without nerves, immunology remains incomplete -in vivo veritas.
Shepherd AJ, Downing JE, Miyan JA. Shepherd AJ, et al. Immunology. 2005 Oct;116(2):145-63. doi: 10.1111/j.1365-2567.2005.02223.x. Immunology. 2005. PMID: 16162264 Free PMC article. Review. - Differential roles of JNK in ConA/GalN and ConA-induced liver injury in mice.
Ni HM, Chen X, Ding WX, Schuchmann M, Yin XM. Ni HM, et al. Am J Pathol. 2008 Oct;173(4):962-72. doi: 10.2353/ajpath.2008.080358. Epub 2008 Sep 4. Am J Pathol. 2008. PMID: 18772342 Free PMC article. - Cytokines, neurokines or both? Mixed mechanisms of mechanical lung injury.
McKechnie SR, Drummond GB. McKechnie SR, et al. J Physiol. 2010 Jun 1;588(Pt 11):1813-4. doi: 10.1113/jphysiol.2010.191478. J Physiol. 2010. PMID: 20516345 Free PMC article. No abstract available.
References
- Helke CJ, Krause JE, Mantyh PW, Couture R, Bannon MJ. Diversity in mammalian tachykinin peptidergic neurons: multiple peptides, receptors, and regulatory mechanisms. FASEB J. 1990;4:1606–1615. - PubMed
- McDonald DM, Bowden JJ, Baluk P, Bunnett NW. Neurogenic inflammation. A model for studying efferent actions of sensory nerves. Adv. Exp. Med. Biol. 1996;410:453–462. - PubMed
- Espiritu RF, Pittet JF, Matthay MA, Goetzl EJ. Neuropeptides in pulmonary edema fluid of adult respiratory distress syndrome. Inflammation. 1992;16:509–517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases