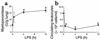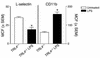Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs - PubMed (original) (raw)
Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs
Graciela Andonegui et al. J Clin Invest. 2003 Apr.
Erratum in
- J Clin Invest. 2003 Oct;112(8):1264
Abstract
The rapid and selective accumulation of neutrophils into the lungs is thought to underlie the pulmonary failure that leads to sepsis-related death. In this study we investigated whether neutrophil TLR4 is important in LPS-induced pulmonary neutrophil recruitment by creating chimeric mice (transferring bone marrow between TLR4(+/+) and TLR4(-/-) mice). In TLR4(+/+) mice receiving TLR4(-/-) bone marrow, 6 weeks after transplant TLR4 was absent in all circulating leukocytes as well as in resident macrophages (these mice were termed LeukocyteTLR4(-/-)), and these cells were completely nonresponsive to LPS. In TLR4(-/-) mice receiving TLR4(+/+) bone marrow, endothelial cells but not leukocytes were deficient in TLR4 (EndotheliumTLR4(-/-)). Surprisingly, systemic LPS (0.5 mg/kg) induced a dramatic increase in neutrophil sequestration into the lungs of LeukocyteTLR4(-/-) mice over the first 4 hours. Concomitantly, numbers of circulating leukocytes decreased by 90%. By contrast, EndotheliumTLR4(-/-) mice showed very little increase in neutrophil sequestration in the lungs, suggesting that endothelium rather than leukocyte TLR4 was important. Intravital microscopy of peripheral microcirculation in the cremaster muscle revealed about 30-fold more leukocyte-endothelial cell interactions in LPS-treated EndotheliumTLR4(-/-) mice than in LPS-treated LeukocyteTLR4(-/-) mice. This is consistent with less sequestration of leukocytes into the lungs of EndotheliumTLR4(-/-) mice. In conclusion, our data challenge the view that LPS directly activates neutrophils to trap in lungs and suggest a far more important role than previously appreciated for the endothelial cells.
Figures
Figure 1
Systemic LPS induces neutrophil sequestration into the lungs and reduces the number of circulating leukocytes in TLR4+/+ mice. Mice were untreated or treated with LPS for 30 minutes, 4 hours, or 12 hours. At these times the lungs were harvested to determine MPO levels (a), and samples of blood were drawn by cardiac puncture to assess the number of circulating leukocytes (b). Data are expressed as the arithmetic mean ± SEM of four to five mice per group. *P < 0.05 vs. untreated mice (0 hours).
Figure 2
LPS-induced neutrophil sequestration into the lung capillaries of TLR4+/+ mice. Mice were treated with LPS for 4 hours, and the lungs were prepared for histology or electron microscopy. (a) Leder (esterase) stain of lung sections. Untreated mice (left panel) show normal alveolar septa with capillaries (small arrows) and small venules (large arrow). No neutrophils are seen in this section. LPS-treated mice (right panel) show infiltrating neutrophils (arrowheads) within capillaries and venules. The inset shows several stained neutrophils in a venule. (b) Quantitative analysis of neutrophil sequestration into the different lung compartments. hpf, high-power field. (c) The electromicrograph shows several neutrophils (arrowheads) within capillaries. *P < 0.05 vs. untreated mice.
Figure 3
Circulating neutrophils exposed to LPS in vivo exhibit a state of activation. TLR4+/+ mice were treated with or without LPS for 4 hours, and then whole blood was drawn by cardiac puncture. Neutrophil L-selectin and CD11b expression were assessed by flow cytometric analysis. Results are shown as the MCF ± SEM for untreated (white bars) and LPS-treated (black bars) mice (n = 3 per group). *P < 0.05 vs. untreated mice.
Figure 4
LPS stimulates neutrophil sequestration into the lungs in E/P-selectin–/– and CD18–/– mice. Mice were treated without LPS (white bars) or with LPS (black bars) for 4 hours, and then whole blood was taken by cardiac puncture and lungs harvested. (a) Circulating-leukocyte counts in harvested lungs. (b) MPO levels in harvested lungs. Data are expressed as the arithmetic mean ± SEM of four mice per group. *P < 0.05 vs. untreated mice.
Figure 5
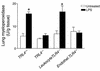
LPS-induced neutrophil sequestration into the lungs of LeukocyteTLR4–/– mice. Mice were untreated (white bars) or treated with LPS for 4 hours (black bars), and then lungs were harvested and MPO levels determined in TLR4+/+, TLR4–/–, LeukocyteTLR4–/–, and EndotheliumTLR4–/– (Endothel.TLR4–/–) chimeric mice. Data are expressed as the arithmetic mean ± SEM of five mice per group and represent experiments pooled from both sets of chimeric mice. *P < 0.05 vs. untreated mice.
Figure 6
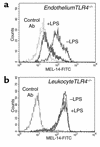
L-selectin shedding from LPS-treated neutrophils differs between TLR4 chimeric mice. Whole blood drawn by cardiac puncture was untreated or treated with LPS for 30 minutes prior to analysis of L-selectin expression by neutrophils from EndotheliumTLR4–/– mice (a) and LeukocyteTLR4–/– mice (b). Flow cytometric analysis is shown for a nonspecific isotype control (broken line), L-selectin expression on untreated cells (thin solid line), and L-selectin expression on LPS-treated cells (heavy solid line). Results shown are representative of three experiments per mouse group using C57BL/6 vs. TLR4–/– chimeric mice.
Figure 7
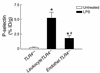
Effect of LPS on P-selectin expression in TLR4 chimeric mice. Expression of P-selectin in lung tissue of untreated TLR4+/+ mice (white bar) and LPS-treated LeukocyteTLR4–/– and EndotheliumTLR4–/– (Endothel.TLR4–/–) chimeric mice (black bars). Data are presented as the arithmetic mean ± SEM of four mice per group and represent experiments using chimeric mice generated with C57BL/6 vs. TLR4–/– mice. *P < 0.05 vs. untreated TLR4+/+ mice; #P < 0.05 vs. LeukocyteTLR4–/– mice. ID, injected dose.
Figure 8
Expression of TLR4 on bone marrow–derived and non-bone-marrow–derived lung macrophages. Macrophages were isolated from EndotheliumTLR4–/– (Endothel.TLR4–/–) and LeukocyteTLR4–/– chimeric mouse lungs, and then treated with LPS for 18 hours or left untreated. Complementary DNA was synthesized, and RT-PCR of iNOS and GAPDH expression was performed. Results shown are representative of three experiments per mouse group using chimeras generated with C57BL/6 and TLR4–/– mice.
Figure 9
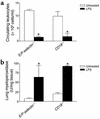
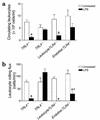
Effect of systemic LPS on circulating leukocytes and leukocyte kinetics in cremaster postcapillary venules in TLR4+/+, TLR4–/–, and TLR4 chimeric mice. (a and b) Circulating leukocytes (a) and leukocyte rolling flux (b) in cremaster tissue of untreated (white bars) and LPS-treated (black bars) TLR4+/+, TLR4–/–, LeukocyteTLR4–/–, and EndotheliumTLR4–/– (Endothel.TLR4–/–) chimeric mice. Data are presented as the arithmetic mean ± SEM of six to eight mice per group and represent experiments using both sets of chimeras. *P < 0.05 vs. untreated mice; #P < 0.05 vs. LPS-treated LeukocyteTLR4–/– mice.
Similar articles
- Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation.
Zhou H, Andonegui G, Wong CH, Kubes P. Zhou H, et al. J Immunol. 2009 Oct 15;183(8):5244-50. doi: 10.4049/jimmunol.0901309. Epub 2009 Sep 28. J Immunol. 2009. PMID: 19786543 - Toll-like receptor-4 signaling mediates pulmonary neutrophil sequestration in response to gram-positive bacterial enterotoxin.
Calkins CM, Barsness K, Bensard DD, Vasquez-Torres A, Raeburn CD, Meng X, McIntyre RC Jr. Calkins CM, et al. J Surg Res. 2002 May 15;104(2):124-30. doi: 10.1006/jsre.2002.6422. J Surg Res. 2002. PMID: 12020131 - Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia.
Tavener SA, Long EM, Robbins SM, McRae KM, Van Remmen H, Kubes P. Tavener SA, et al. Circ Res. 2004 Oct 1;95(7):700-7. doi: 10.1161/01.RES.0000144175.70140.8c. Epub 2004 Sep 9. Circ Res. 2004. PMID: 15358664 - Identification of Toll-like receptor 4 (Tlr4) as the sole conduit for LPS signal transduction: genetic and evolutionary studies.
Beutler B, Du X, Poltorak A. Beutler B, et al. J Endotoxin Res. 2001;7(4):277-80. J Endotoxin Res. 2001. PMID: 11717581 Review. No abstract available. - Mechanisms of leukocyte sequestration in inflamed lungs.
Doerschuk CM. Doerschuk CM. Microcirculation. 2001 Apr;8(2):71-88. Microcirculation. 2001. PMID: 11379793 Review.
Cited by
- Toll-Like Receptor 4 Is an Essential Upstream Regulator of On-Time Parturition and Perinatal Viability in Mice.
Wahid HH, Dorian CL, Chin PY, Hutchinson MR, Rice KC, Olson DM, Moldenhauer LM, Robertson SA. Wahid HH, et al. Endocrinology. 2015 Oct;156(10):3828-41. doi: 10.1210/EN.2015-1089. Epub 2015 Jul 7. Endocrinology. 2015. PMID: 26151355 Free PMC article. - Ensemble models of neutrophil trafficking in severe sepsis.
Song SO, Song SO, Hogg J, Peng ZY, Parker R, Kellum JA, Clermont G. Song SO, et al. PLoS Comput Biol. 2012;8(3):e1002422. doi: 10.1371/journal.pcbi.1002422. Epub 2012 Mar 8. PLoS Comput Biol. 2012. PMID: 22412365 Free PMC article. - Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation.
Rossaint J, Margraf A, Zarbock A. Rossaint J, et al. Front Immunol. 2018 Nov 20;9:2712. doi: 10.3389/fimmu.2018.02712. eCollection 2018. Front Immunol. 2018. PMID: 30515177 Free PMC article. Review. - Association of Toll-like receptor signaling and reactive oxygen species: a potential therapeutic target for posttrauma acute lung injury.
Xiang M, Fan J, Fan J. Xiang M, et al. Mediators Inflamm. 2010;2010:916425. doi: 10.1155/2010/916425. Epub 2010 Jul 13. Mediators Inflamm. 2010. PMID: 20706658 Free PMC article. Review. - Necroptosis triggers spatially restricted neutrophil-mediated vascular damage during lung ischemia reperfusion injury.
Li W, Terada Y, Tyurina YY, Tyurin VA, Bery AI, Gauthier JM, Higashikubo R, Tong AY, Zhou D, Nunez-Santana F, Lecuona E, Hassan A, Hashimoto K, Scozzi D, Puri V, Nava RG, Krupnick AS, Lavine KJ, Gelman AE, Miller MJ, Kagan VE, Bharat A, Kreisel D. Li W, et al. Proc Natl Acad Sci U S A. 2022 Mar 8;119(10):e2111537119. doi: 10.1073/pnas.2111537119. Epub 2022 Mar 1. Proc Natl Acad Sci U S A. 2022. PMID: 35238643 Free PMC article.
References
- Bone RC. The pathogenesis of sepsis. Ann. Intern. Med. 1991;115:457–469. - PubMed
- Mortality pattern: United States 1990. Centers for Disease Control and Prevention. National Center for Health Statistics. Mon. Vital Stat. Rep. 1993;41:5.
- Welbourn CR, Young Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br. J. Surg. 1992;79:998–1003. - PubMed
- Sheridan BC, et al. Neutrophils mediate pulmonary vasomotor dysfunction in endotoxin-induced acute lung injury. J. Trauma. 1997;42:391–396. - PubMed
- Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases