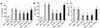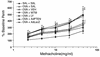Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma - PubMed (original) (raw)
Involvement of PTEN in airway hyperresponsiveness and inflammation in bronchial asthma
Yong-Geun Kwak et al. J Clin Invest. 2003 Apr.
Abstract
Phosphatase and tensin homologue deleted on chromosome ten (PTEN) is part of a complex signaling system that affects a variety of important cell functions. PTEN blocks the action of PI3K by dephosphorylating the signaling lipid phosphatidylinositol 3,4,5-triphosphate. We have used a mouse model for asthma to determine the effect of PI3K inhibitors and PTEN on allergen-induced bronchial inflammation and airway hyperresponsiveness. PI3K activity increased significantly after allergen challenge. PTEN protein expression and PTEN activity were decreased in OVA-induced asthma. Immunoreactive PTEN localized in epithelial layers around the bronchioles in control mice. However, this immunoreactive PTEN dramatically disappeared in allergen-induced asthmatic lungs. The increased IL-4, IL-5, and eosinophil cationic protein levels in bronchoalveolar lavage fluids after OVA inhalation were significantly reduced by the intratracheal administration of PI3K inhibitors or adenoviruses carrying PTEN cDNA (AdPTEN). Intratracheal administration of PI3K inhibitors or AdPTEN remarkably reduced bronchial inflammation and airway hyperresponsiveness. These findings indicate that PTEN may play a pivotal role in the pathogenesis of the asthma phenotype.
Figures
Figure 1
Schematic diagram of the experimental protocol. Mice were sensitized on days 1 and 14 by intraperitoneal injection of OVA emulsified in 1 mg aluminum hydroxide. On days 21, 22, and 23 after the initial sensitization, the mice were challenged for 30 minutes with an aerosol of 1% (wt/vol) OVA in saline (or with saline as a control) using an ultrasonic nebulizer. Wortmannin, LY-294002, or Ad vector was administered intratracheally two times to each treated animal, once on day 21 (1 hour before the first airway challenge with OVA) and the second time on day 23 (3 hours after the last airway challenge with OVA).
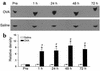
Figure 2
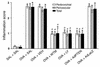
Inducible PI3K enzyme activity in lung tissues of OVA-sensitized and -challenged mice. (a) PI3K activity was measured in lung tissues from sensitized mice challenged with OVA (upper panel) or with saline (lower panel). (b) Results of densitometric analysis are presented as the relative ratio of induction of PI3K before and after the challenge. The PI3K activity in the lung tissue of preinhalation mice is arbitrarily presented as 1. Data represent mean ± SD from six independent experiments. Pre, before challenge; 1, 24, 48, and 72 hours are time periods after the last challenge. *P < 0.05 vs. Pre; #P < 0.05 vs. saline inhalation.
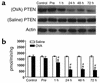
Figure 3
Western blot analyses of PTEN protein and PTEN activity in lung tissues of OVA-sensitized and -challenged mice. (a) Western blot analyses of PTEN protein. Sampling was performed in lung tissues from sensitized mice challenged with OVA or saline. The Western blot was probed with an anti-PTEN antibody and reprobed with an anti-actin antibody to verify equal loading of protein in each lane. Results were similar in six independent experiments. (b) PTEN activity was measured in lung tissues from sensitized mice challenged with OVA or with saline. Data are presented as mean ± SD from six independent experiments. *P < 0.05 vs. Pre; #P < 0.05 vs. saline inhalation. Control, no treatment.
Figure 4
Effect of wortmannin, LY-294002, or AdPTEN on p-Akt and Akt protein expression in lung tissues of OVA-sensitized and -challenged mice. p-Akt and Akt protein expression were measured 72 hours after the last challenge in saline-inhaled mice administered saline intratracheal ly (SAL + SAL), OVA-inhaled mice administered saline (OVA + SAL), OVA-inhaled mice administered drug vehicle (OVA + VEH), OVA-inhaled mice administered wortmannin (OVA + WTM), OVA-inhaled mice administered LY-294002 (OVA + LY), OVA-inhaled mice administered AdPTEN (OVA + AdPTEN), and OVA-inhaled mice administered AdLacZ (OVA + AdLacZ). Results were similar in six independent experiments.
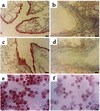
Figure 5
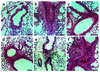
Localization of immunoreactive PTEN in lung tissues and tracheal epithelial cells of OVA-sensitized and -challenged mice. Sampling was performed 72 hours after the last challenge in lung tissues and tracheal epithelial cells from sensitized mice challenged with saline (a and e), from sensitized mice challenged with OVA (b and f), from OVA-inhaled mice administered AdPTEN (c), and from OVA-inhaled mice administered AdLacZ (d). Representative light microscopy shows PTEN-positive cells in the bronchioles (a, b, c, and d; the brown color indicates PTEN-positive cells) and in the tracheal epithelial cells (e and f; the dark brown color indicates PTEN-positive cells). Bars indicate scale of 50 μm (a, b, c, and d) or 10 μm (e and f).
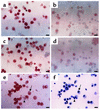
Figure 6
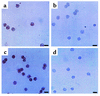
Localization of immunoreactive PTEN in BAL fluids of OVA-sensitized and -challenged mice. Sampling was performed 72 hours after the last challenge in BAL fluids from sensitized mice challenged with saline (a, e, and f), sensitized mice challenged with OVA (b), OVA-inhaled mice administered AdPTEN (c), and OVA-inhaled mice administered AdLacZ (d). Representative light microscopy shows PTEN-positive cells in the BAL fluids (a, b, c, d, and e); the brown color indicates PTEN-positive cells. (f) To examine the cell differentials in BAL cells prepared from the control mice, the slides used for the detection of PTEN (e) were destained with 70% ethyl alcohol. The smears of BAL cells were stained with Diff-Quik solution and viewed under a light microscope. The arrow indicates a macrophage; the arrowhead indicates a lymphocyte. Bars indicate 10 μm.
Figure 7
Representative photomicrographs showing localization of immunoreactive PTEN in BAL fluid eosinophils of OVA-sensitized and -challenged mice. Sampling was performed 72 hours after the last challenge in BAL fluids from sensitized mice challenged with saline (a), from sensitized mice challenged with OVA (b), from OVA-inhaled mice administered AdPTEN (c), and from OVA-inhaled mice administered AdLacZ (d). Representative light microscopy of PTEN-positive cells in the BAL eosinophils; the brown color indicates PTEN-positive cells. Bars indicate 10 μm.
Figure 8
Localization of immunoreactive β-gal in lung tissues and BAL fluid eosinophils of OVA-sensitized and -challenged mice. Sampling was performed 72 hours after the last challenge in lung tissues from OVA-inhaled mice administered AdLacZ (a) and sensitized mice challenged with OVA (b). Blue-stained cells were considered to express the LacZ gene. Bars indicate 10 μm (a and b). Percentage of β-gal–stained cells in BAL fluid eosinophils (c). Sampling was performed at 24, 48, and 72 hours after the last challenge in BAL fluids from OVA-inhaled mice administered AdLacZ. Data represent mean ± SD from six independent experiments.
Figure 9
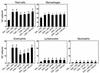
Effect of wortmannin, LY-294002, or AdPTEN on total and differential cellular components of BAL. Shown are numbers of each cellular component of BAL from mice treated as described in Figure 4 legend, counted 72 hours after the last challenge. Bars represent mean ± SD from six independent experiments. *P < 0.05 vs. SAL + SAL; #P < 0.05 vs. OVA + SAL and OVA + AdLacZ.
Figure 10
Effect of wortmannin, LY-294002, or AdPTEN in lung tissues of OVA-sensitized and -challenged mice. Representative H&E-stained sections of the lungs. Sampling was performed 72 hours after the last challenge in saline-inhaled mice administered saline (a), OVA-inhaled mice administered saline (b), OVA-inhaled mice administered wortmannin (c), OVA-inhaled mice administered LY-294002 (d), OVA-inhaled mice administered AdPTEN (e), and OVA-inhaled mice administered AdLacZ (f). Bars indicate scale of 50 μm.
Figure 11
Effect of wortmannin, LY-294002, or AdPTEN on peribronchial and perivascular lung inflammation. Peribronchial, perivascular, and total lung inflammation were measured 72 hours after the last challenge in mice treated as described in Figure 4 legend. Bars represent mean ± SD from six independent experiments. Total lung inflammation was defined as the average of the peribronchial and perivascular inflammation scores. *P < 0.05 vs. SAL + SAL; #P < 0.05 vs. OVA + SAL and OVA + AdLacZ.
Figure 12
Effect of wortmannin, LY-294002, or AdPTEN on IL-4, IL-5, and ECP levels in BAL fluids of OVA-sensitized and -challenged mice. Enzyme immunoassay of IL-4 (a), IL-5 (b), and ECP (c). Sampling was performed 72 hours after the last challenge in mice treated as described in Figure 4 legend. Bars represent mean ± SD from six independent experiments. *P < 0.05 vs. SAL + SAL; #P < 0.05 vs. OVA + SAL and OVA + AdLacZ.
Figure 13
Effect of wortmannin, LY-294002, or AdPTEN on airway responsiveness in OVA-sensitized and -challenged mice. Airway responsiveness was measured 72 hours after the last challenge in mice treated as described in Figure 4 legend. Airway responsiveness to aerosolized methacholine was measured in unrestrained, conscious mice. Mice were placed in the main chamber of a barometric plethysmograph and nebulized first with saline and then with increasing doses (from 2.5 to 50 mg/ml) of methacholine for 3 minutes for each nebulization. Readings of breathing parameters were taken for 3 minutes after each nebulization, during which time Penh values were determined. Data represent mean ± SD from six independent experiments. *P < 0.05 vs. SAL + SAL; #P < 0.05 vs. OVA + SAL and OVA + AdLacZ.
Similar articles
- Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) reduces vascular endothelial growth factor expression in allergen-induced airway inflammation.
Lee KS, Kim SR, Park SJ, Lee HK, Park HS, Min KH, Jin SM, Lee YC. Lee KS, et al. Mol Pharmacol. 2006 Jun;69(6):1829-39. doi: 10.1124/mol.106.022228. Epub 2006 Mar 9. Mol Pharmacol. 2006. PMID: 16527906 - The role of PTEN in allergic inflammation.
Lee YC. Lee YC. Arch Immunol Ther Exp (Warsz). 2004 Jul-Aug;52(4):250-4. Arch Immunol Ther Exp (Warsz). 2004. PMID: 15467489 Review. - PPAR-gamma modulates allergic inflammation through up-regulation of PTEN.
Lee KS, Park SJ, Hwang PH, Yi HK, Song CH, Chai OH, Kim JS, Lee MK, Lee YC. Lee KS, et al. FASEB J. 2005 Jun;19(8):1033-5. doi: 10.1096/fj.04-3309fje. Epub 2005 Mar 23. FASEB J. 2005. PMID: 15788448 - PTEN down-regulates IL-17 expression in a murine model of toluene diisocyanate-induced airway disease.
Kim SR, Lee KS, Park SJ, Min KH, Lee KY, Choe YH, Lee YR, Kim JS, Hong SJ, Lee YC. Kim SR, et al. J Immunol. 2007 Nov 15;179(10):6820-9. doi: 10.4049/jimmunol.179.10.6820. J Immunol. 2007. PMID: 17982072 - Pten signaling in gliomas.
Knobbe CB, Merlo A, Reifenberger G. Knobbe CB, et al. Neuro Oncol. 2002 Jul;4(3):196-211. Neuro Oncol. 2002. PMID: 12084351 Free PMC article. Review.
Cited by
- Caught in the Akt: regulation of Wnt signaling in the intestine.
Anderson EC, Wong MH. Anderson EC, et al. Gastroenterology. 2010 Sep;139(3):718-22. doi: 10.1053/j.gastro.2010.07.012. Epub 2010 Jul 24. Gastroenterology. 2010. PMID: 20659460 Free PMC article. No abstract available. - Defining Bronchial Asthma with Phosphoinositide 3-Kinase Delta Activation: Towards Endotype-Driven Management.
Jeong JS, Kim JS, Kim SR, Lee YC. Jeong JS, et al. Int J Mol Sci. 2019 Jul 18;20(14):3525. doi: 10.3390/ijms20143525. Int J Mol Sci. 2019. PMID: 31323822 Free PMC article. Review. - Hydrogen Sulfide Downregulates Oncostatin M Expression via PI3K/Akt/NF-κB Signaling Processes in Neutrophil-like Differentiated HL-60 Cells.
Han NR, Ko SG, Park HJ, Moon PD. Han NR, et al. Antioxidants (Basel). 2023 Feb 8;12(2):417. doi: 10.3390/antiox12020417. Antioxidants (Basel). 2023. PMID: 36829975 Free PMC article. - Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT.
Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, Liu J, Munoz NM, Zhu X. Myou S, et al. J Exp Med. 2003 Nov 17;198(10):1573-82. doi: 10.1084/jem.20030298. J Exp Med. 2003. PMID: 14623911 Free PMC article. - PTEN gene silencing contributes to airway remodeling and induces airway smooth muscle cell proliferation in mice with allergic asthma.
Wen X, Yan J, Han XR, Zheng GH, Tang R, Liu LF, Wu DM, Lu J, Zheng YL. Wen X, et al. J Thorac Dis. 2018 Jan;10(1):202-211. doi: 10.21037/jtd.2017.12.104. J Thorac Dis. 2018. PMID: 29600050 Free PMC article. Retracted.
References
- Kay AB. Asthma and inflammation. J. Allergy Clin. Immunol. 1991;87:893–910. - PubMed
- Frigas E, Gleich GJ. The eosinophil and the pathophysiology of asthma. J. Allergy Clin. Immunol. 1986;77:527–537. - PubMed
- Dunzendorfer S, Meierhofer C, Wiedermann CJ. Signaling in neuropeptide-induced migration of human eosinophils. J. Leukoc. Biol. 1998;64:828–834. - PubMed
- Zhu X, et al. A surrogate method for assessment of beta (2)-integrin-dependent adhesion of eosinophils to ICAM-1. J. Immunol. Meth. 2000;240:157–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials