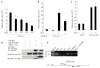A glucocorticoid-induced leucine-zipper protein, GILZ, inhibits adipogenesis of mesenchymal cells - PubMed (original) (raw)
A glucocorticoid-induced leucine-zipper protein, GILZ, inhibits adipogenesis of mesenchymal cells
Xingming Shi et al. EMBO Rep. 2003 Apr.
Abstract
Mesenchymal stem cells have the potential to differentiate into various cell lineages, including adipocytes and osteoblasts. The induction of adipocyte differentiation by glucocorticoids (GCs) not only causes the accumulation of fat cells in bone marrow, but also depletes the supply of osteoblasts for new bone formation, thus leading to osteoporosis. We have shown that a GC-induced leucine-zipper protein (GILZ) antagonizes adipocyte differentiation. GILZ binds to a tandem repeat of CCAAT/enhancer-binding protein (C/EBP) binding sites in the promoter of the gene encoding peroxisome-proliferator-activated receptor-gamma2 (PPAR-gamma2), and inhibits its transcription as a sequence-specific transcriptional repressor. We have also shown that ectopic expression of GILZ blocks GC-induced adipocyte differentiation. Furthermore, adipogenic marker genes (for example, those encoding PPAR-gamma2, C/EBP-alpha, lipoprotein lipase and adipsin) are also inhibited by GILZ. Our results reveal a novel GC antagonistic mechanism that has potential therapeutic applications for the inhibition of GC-induced adipocyte differentiation.
Figures
Figure 1
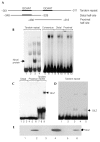
The novel glucocorticoid-induced nuclear protein that binds to the peroxisome-proliferator-activated receptor-γ2 promoter is the glucocorticoid-induced leucine-zipper protein, GILZ. (A) Representation of peroxisome-proliferator-activated receptor-γ2 (PPAR-γ2) promoter fragments used as probes. (B) Electrophoretic mobility-shift assays, showing the binding of a glucocorticoid (GC)-induced nuclear protein to the tandem repeat of CCAAT/enhancer-binding protein (C/EBP) binding sites in the PPAR-γ2 promoter. Nuclear proteins from untreated (lanes 2, 6, 9 and 12), dexamethasone (Dex)-treated (lanes 3, 7, 10 and 13) or Dex/cycloheximide-treated (lane 4) C3H10T1/2 cells were incubated with the labelled probes shown in (A) and a high-affinity consensus C/EBP binding site from the C/EBP-α promoter (lanes 5–7). Lanes 1, 5, 8 and 11 contained no protein. (C) Electrophoretic mobility-shift assays, showing the specific binding of the glutathione-_S_-transferase (GST)–GILZ fusion protein to the tandem repeats of C/EBP binding sites. Lanes 1, 4 and 7 contained no protein. Lanes 2, 5 and 8 contained 5 μg of GST. Lanes 3, 6 and 9 contained 5 μg of the GST–GILZ fusion protein. (D) Overexpressed GILZ binds to a tandem-repeat probe. COS1 cells were transfected with empty pcDNA3 or with the pcDNA3–GILZ expression plasmid, and whole-cell lysates were incubated with a labelled tandem-repeat probe and used in electrophoretic mobility-shift assays. Lane 1, no protein. Lanes 2 and 3, nuclear protein from untreated and Dex-treated C3H10T1/2 cells, respectively. Lanes 4 and 5, whole-cell lysates from pcDNA3 and pcDNA3–GILZ-transfected COS1 cells. (E) Western blot analysis using an anti-GILZ antibody to detect proteins from the following samples: cell lysates from C3H10T1/2 cells, either untreated (lane 1) or treated with Dex (lane 2); COS1 cell lysates transfected with an empty pcDNA3 vector (lane 3) or with the pcDNA3–GILZ expression plasmid (lane 4); and nuclear proteins from C3H10T1/2 cells, either untreated (lane 5) or treated with Dex (lane 6).
Figure 2
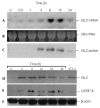
Glucocorticoid-induced leucine-zipper protein messenger RNA and protein expression are induced by glucocorticoid in a time-dependent manner. (A–C) C3H10T1/2 cells were treated with dexamethasone (Dex), and total RNA and protein were prepared at the time points indicated. Levels of glucocorticoid-induced leucine-zipper protein (GILZ) mRNA (A) and protein (C) expression were determined by northern and western blot analyses, using a randomly primed GILZ complementary DNA probe and an anti-GILZ antibody, respectively. Equal loading of lanes is shown by ethidium bromide staining of 28S ribosomal RNA (B). (D–F) 3T3-L1 cells were treated with 100 nM of Dex, and the whole-cell lysates were prepared at the time points indicated. The levels of GILZ protein (D) were determined by western blot analysis using an anti-GILZ antibody. A sample from a stable cell line (3T3-Z) was also included to show the expression level of GILZ protein in these cells. (E) The same membrane as in (D) was stripped and re-probed with anti-CCAAT/enhancer-binding protein-δ (C/EBP-δ) antibody to show the levels of C/EBP-δ expression obtained in response to stimulation with Dex. Equal loading of lanes is shown by assaying levels of β-actin expression (F).
Figure 3
The glucocorticoid-induced leucine-zipper protein represses CCAAT/enhancer-binding-protein-δ-mediated transcription of the gene encoding peroxisome-proliferator-activated receptor-γ2. (A,B) A wild-type peroxisome-proliferator-activated receptor-γ2 (PPAR-γ2) promoter–luciferase reporter (615-Luc) or (C) a mutant, which has a 22-nucleotide deletion of the region containing the tandem repeat of the CCAAT/enhancer-binding protein (C/EBP) binding sites (615-Luc-del), were co-transfected with a glucocorticoid-induced leucine-zipper protein (GILZ) expression vector, or with both GILZ and C/EBP-δ expression vectors, into C3H10T1/2 cells. The cells were incubated for 48 h before luciferase activities were measured. The empty luciferase reporter vector, pGL3-basic (pGL3), was used as a control (C). The experiment in panel (A) was performed in 12-well plates with 400 ng per well of reporter plasmid; and 24-well plates were used in (B) and (C) with 200 ng of reporter plasmid and 100 ng of the pcDNA3–GILZ plasmid. MSV–EBP-δ (C/EBP-δ) plasmid (50 ng) was used in (B). The values were normalized to a Renilla luciferase internal control. Representative examples of results from many experiments, carried out in triplicate, are shown. (D) GILZ interacts with histone deacetylase 1 (HDAC1). Flag-epitope-tagged GILZ and haemagglutinin (HA)-tagged HDAC1 expression plasmids were co-transfected into COS1 cells. Cell lysates were immunoprecipitated using an anti-Flag antibody, and the immunoprecipitates were analysed by western blotting using an anti-HA antibody (upper panel). The interaction of SMAD6C and HDAC1 is shown as a positive control (upper panel, lane 4). The two lower panels show western blots of cell lysates, indicating the relative expression levels of the corresponding proteins. (E) Chromatin immunoprecipitation (ChIP) assay indicating the in vivo interaction between GILZ and C/EBP-δ at the PPAR-γ2 promoter region. 3T3-L1 cells were treated with 100 nM of dexamethasone for 12 h, after which the ChIP assays were carried out (see Methods) using anti-HA, anti-GILZ and anti-C/EBP-δ antibodies, as indicated. (F) Location of primers from the PPAR-γ2 promoter region used in the ChIP assay in (E) to amplify target DNA by PCR. The size of the predicted PCR product was 167 bp.
Figure 4
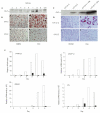
The glucocorticoid-induced leucine-zipper protein inhibits adipocyte differentiation of 3T3-L1 and C3H10T1/2 cells. (A) Western blot analysis of glucocorticoid-induced leucine-zipper protein (GILZ) expression in 3T3-L1 stable cell lines. Lysates from 3T3-Z (lanes 2–9) and 3T3-C (Ctrl) cells were analysed by western blotting using an anti-GILZ antibody. (B) The control (3T3-C) and GILZ-expressing (3T3-Z) cells were cultured in DMEM (a, c) or adipocyte induction medium (MID) containing isobutylmethylxanthine (0.5 mM), insulin (10 μg ml−1) and dexamethasone (Dex; 1 μM) (b, d). The cells were stained with Oil-Red O 5 days after induction. The red colour indicates the lipid droplets in adipocytes. (C) Western blot analysis of GILZ expression, carried out as for (A), in C3H10T1/2 stably transfected cells. Lanes 3 and 4 show the levels of GILZ in cells treated with Dex. (D) Control (10T1/2-C) and GILZ-expressing (10T1/2-Z) cells were cultured in DMEM (a, c) or treated with 1 μM Dex (b, d) for 12 days, and were then stained as in (B). (E) Real-time RT–PCR (reverse transcription followed by PCR) analysis of adipocyte marker gene expression during adipocyte differentiation. An adipocyte differentiation procedure was carried out on stably transfected 3T3-C (empty bars) and 3T3-Z (filled bars) cell lines. Total RNAs were isolated at the timepoints indicated, and messenger RNA levels for PPAR-γ2, C/EBP-α, adipsin and lipoprotein lipase (LPL) were determined by real-time RT-PCR. The levels of mRNA were normalized to that of β-actin, and the values from 3T3-C cells (control) for each gene at day 0 were set as 1. Two independent experiments were carried out, and one representative experimental result is shown. Note that the scales used to show mRNA levels vary for the different genes analysed.
Similar articles
- Tandem repeat of C/EBP binding sites mediates PPARgamma2 gene transcription in glucocorticoid-induced adipocyte differentiation.
Shi XM, Blair HC, Yang X, McDonald JM, Cao X. Shi XM, et al. J Cell Biochem. 2000 Jan;76(3):518-27. doi: 10.1002/(sici)1097-4644(20000301)76:3<518::aid-jcb18>3.0.co;2-m. J Cell Biochem. 2000. PMID: 10649448 - Regulation of mesenchymal stem cell osteogenic differentiation by glucocorticoid-induced leucine zipper (GILZ).
Zhang W, Yang N, Shi XM. Zhang W, et al. J Biol Chem. 2008 Feb 22;283(8):4723-9. doi: 10.1074/jbc.M704147200. Epub 2007 Dec 14. J Biol Chem. 2008. PMID: 18084007 - Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins.
Yeh WC, Cao Z, Classon M, McKnight SL. Yeh WC, et al. Genes Dev. 1995 Jan 15;9(2):168-81. doi: 10.1101/gad.9.2.168. Genes Dev. 1995. PMID: 7531665 - Glucocorticoid-induced leucine zipper (GILZ): a new important mediator of glucocorticoid action.
Ayroldi E, Riccardi C. Ayroldi E, et al. FASEB J. 2009 Nov;23(11):3649-58. doi: 10.1096/fj.09-134684. Epub 2009 Jun 30. FASEB J. 2009. PMID: 19567371 Review. - Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma.
Tontonoz P, Hu E, Spiegelman BM. Tontonoz P, et al. Curr Opin Genet Dev. 1995 Oct;5(5):571-6. doi: 10.1016/0959-437x(95)80025-5. Curr Opin Genet Dev. 1995. PMID: 8664544 Review.
Cited by
- Induction of Glucocorticoid-induced Leucine Zipper (GILZ) Contributes to Anti-inflammatory Effects of the Natural Product Curcumin in Macrophages.
Hoppstädter J, Hachenthal N, Valbuena-Perez JV, Lampe S, Astanina K, Kunze MM, Bruscoli S, Riccardi C, Schmid T, Diesel B, Kiemer AK. Hoppstädter J, et al. J Biol Chem. 2016 Oct 28;291(44):22949-22960. doi: 10.1074/jbc.M116.733253. Epub 2016 Sep 14. J Biol Chem. 2016. PMID: 27629417 Free PMC article. - Tomorrow's skeleton staff: mesenchymal stem cells and the repair of bone and cartilage.
Otto WR, Rao J. Otto WR, et al. Cell Prolif. 2004 Feb;37(1):97-110. doi: 10.1111/j.1365-2184.2004.00303.x. Cell Prolif. 2004. PMID: 14871240 Free PMC article. Review. - Role of GILZ in immune regulation, glucocorticoid actions and rheumatoid arthritis.
Beaulieu E, Morand EF. Beaulieu E, et al. Nat Rev Rheumatol. 2011 Jun;7(6):340-8. doi: 10.1038/nrrheum.2011.59. Epub 2011 May 10. Nat Rev Rheumatol. 2011. PMID: 21556028 Review. - Glucocorticoid-induced leucine zipper (GILZ) and long GILZ inhibit myogenic differentiation and mediate anti-myogenic effects of glucocorticoids.
Bruscoli S, Donato V, Velardi E, Di Sante M, Migliorati G, Donato R, Riccardi C. Bruscoli S, et al. J Biol Chem. 2010 Apr 2;285(14):10385-96. doi: 10.1074/jbc.M109.070136. Epub 2010 Feb 2. J Biol Chem. 2010. PMID: 20124407 Free PMC article. - Protein phosphatase 5 mediates lipid metabolism through reciprocal control of glucocorticoid receptor and peroxisome proliferator-activated receptor-γ (PPARγ).
Hinds TD Jr, Stechschulte LA, Cash HA, Whisler D, Banerjee A, Yong W, Khuder SS, Kaw MK, Shou W, Najjar SM, Sanchez ER. Hinds TD Jr, et al. J Biol Chem. 2011 Dec 16;286(50):42911-22. doi: 10.1074/jbc.M111.311662. Epub 2011 Oct 12. J Biol Chem. 2011. PMID: 21994940 Free PMC article.
References
- Ayroldi E., Migliorati G., Bruscoli S., Marchetti C., Zollo O., Cannarile L., D'Adamio F. & Riccardi C. (2001) Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor κB. Blood, 98, 743–753. - PubMed
- Bellows C.G., Aubin J.E. & Heersche J.N. (1987) Physiological concentrations of glucocorticoids stimulate formation of bone nodules from isolated rat calvaria cells in vitro. Endocrinology, 121, 1985–1992. - PubMed
- Bellows C.G., Ciaccia A. & Heersche J.N. (1998) Osteoprogenitor cells in cell populations derived from mouse and rat calvaria differ in their response to corticosterone, cortisol and cortisone. Bone, 23, 119–125. - PubMed
- Christy R.J., Yang V.W., Ntambi J.M., Geiman D.E., Landschulz W.H., Friedman A.D., Nakabeppu Y., Kelly T.J. & Lane M.D. (1989) Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocytespecific genes. Genes Dev., 3, 1323–1335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous