Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules - PubMed (original) (raw)
. 2003 Apr 7;88(7):1004-11.
doi: 10.1038/sj.bjc.6600827.
S L Gerson, L Denis, C Geyer, L A Hammond, A Patnaik, A D Goetz, G Schwartz, T Edwards, L Reyderman, P Statkevich, D L Cutler, E K Rowinsky
Affiliations
- PMID: 12671695
- PMCID: PMC2376384
- DOI: 10.1038/sj.bjc.6600827
Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules
A W Tolcher et al. Br J Cancer. 2003.
Abstract
Temozolomide, an oral DNA methylator that inactivates the DNA repair enzyme O(6)-alkylguanine-DNA alkyltransferase (AGAT), has demonstrated anticancer activity on protracted schedules. Protracted schedules may lead to an 'autoenhancement' of temozolomide's inherent cytotoxic potential by cumulative reduction of the cell's capacity for AGAT-mediated DNA repair and resistance. This study was undertaken to characterise AGAT inactivation and regeneration in the peripheral blood mononuclear cells (PBMCs) of patients treated on two protracted temozolomide schedules. O(6)-alkyl guanine-DNA alkyltransferase activity was measured in the PBMCs of patients treated on two phase I protracted temozolomide studies. Patients were treated daily for either 7 days every 2 weeks (Schedule A) or 21 days every 4 weeks (Schedule B). The effects of various temozolomide doses (75-175 mg m(-2)), treatment duration (7-21 days), and temozolomide plasma levels on AGAT inactivation and regeneration, as well as the relation between AGAT inactivation and toxicity, were studied. O(6)-alkyl guanine-DNA alkyltransferase activity in PBMCs was measured serially in 52 patients. Marked inactivation of AGAT occurred following 7 days of temozolomide treatment, with mean AGAT activity decreasing by 72% (P<0.0001). Similarly, mean AGAT activity decreased by 63 and 73% after 14 and 21 days of treatment, respectively (P<0.001 for both comparisons). O(6)-alkyl guanine-DNA alkyltransferase inactivation was greater after 7 days of treatment with higher doses of temozolomide than lower doses and remained markedly reduced 7 days post-treatment. However, AGAT inactivation following temozolomide treatment for 14 and 21 days was similar at all doses. On the continuous 21-day schedule, AGAT inactivation was significantly greater in patients who experienced severe thrombocytopenia than those who did not (90.3+/-5.5 vs 72.5+/-16.1%, P<0.045). In conclusion, protracted administration of temozolomide, even at relatively low daily doses, leads to significant and prolonged depletion of AGAT activity, which may enhance the antitumour activity of the agent.
Figures
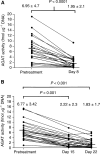
Figure 1
Scatterplots of AGAT activity in PBMCs sampled (A), pretreatment and after 7 days of daily temozolomide treatment administered (at doses of 75–175 mg m−2 day−1) on Schedule A; and (B) after 14 and 21 days of temozolomide treatment (at doses of 85–125 mg m−2 day−1) on Schedule B.
Figure 2
Scatterplots of the percentage values of PBMC AGAT inactivation for Schedules A (day 8 values) and B (day 15 and 22 values) at each temozolomide dose: (A) 75 mg m−2 day−1 (day 8 only) and 85 mg m−2 day−1 (days 15 and 22); (B) 100 mg m−2 day−1; (C) 125 mg m−2 day−1; (D) 150 mg m−2 day−1; and (E) 175 mg m−2 day−1. Dose-limiting toxicity precluded daily treatment with temozolomide for longer than 7 days at the 150 and 175 mg m−2 day−1 dose levels.
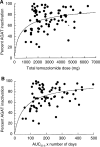
Figure 3
(A) Scatterplot of the percentage values of PBMC AGAT inactivation as a function of the total cumulative temozolomide dose administered up to the time of PBMC sampling (_R_2=0.102). (B) Scatterplot of temozolomide exposure as the product of AUC0-t multiplied by total days temozolomide administered prior to PBMC sampling (_R_2=0.188).
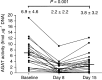
Figure 4
Mean (s.d.) AGAT activity at baseline and day 8, and recovery at day 15 following administration of temozolomide on days 1–8.
Similar articles
- Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O(6)-alkylguanine-DNA-alkyltransferase.
Spiro TP, Liu L, Majka S, Haaga J, Willson JK, Gerson SL. Spiro TP, et al. Clin Cancer Res. 2001 Aug;7(8):2309-17. Clin Cancer Res. 2001. PMID: 11489806 Clinical Trial. - Four-hourly scheduling of temozolomide improves tumour growth delay but not therapeutic index in A375M melanoma xenografts.
Middleton MR, Kelly J, Goodger S, Thatcher N, Margison GP. Middleton MR, et al. Cancer Chemother Pharmacol. 2000;45(1):15-20. doi: 10.1007/PL00006737. Cancer Chemother Pharmacol. 2000. PMID: 10647496 - Effect of single and multiple administration of an O6-benzylguanine/temozolomide combination: an evaluation in a human melanoma xenograft model.
Wedge SR, Porteous JK, Newlands ES. Wedge SR, et al. Cancer Chemother Pharmacol. 1997;40(3):266-72. doi: 10.1007/s002800050657. Cancer Chemother Pharmacol. 1997. PMID: 9219512 - Inactivation of O6-alkylguanine DNA alkyltransferase as a means to enhance chemotherapy.
Rabik CA, Njoku MC, Dolan ME. Rabik CA, et al. Cancer Treat Rev. 2006 Jun;32(4):261-76. doi: 10.1016/j.ctrv.2006.03.004. Epub 2006 May 15. Cancer Treat Rev. 2006. PMID: 16698182 Review.
Cited by
- Radiotherapy and temozolomide for anaplastic astrocytic gliomas.
Nayak L, Panageas KS, Reiner AS, Huse JT, Pentsova E, Braunthal SG, Abrey LE, DeAngelis LM, Lassman AB. Nayak L, et al. J Neurooncol. 2015 May;123(1):129-34. doi: 10.1007/s11060-015-1771-8. Epub 2015 Apr 29. J Neurooncol. 2015. PMID: 25920709 Free PMC article. Clinical Trial. - Dose-dense temozolomide: is it still promising?
Nagane M. Nagane M. Neurol Med Chir (Tokyo). 2015;55(1):38-49. doi: 10.2176/nmc.ra.2014-0277. Epub 2014 Dec 20. Neurol Med Chir (Tokyo). 2015. PMID: 25744349 Free PMC article. Review. - Primary spinal anaplastic ependymoma: A single-institute retrospective cohort and systematic review.
Wu L, Wang L, Zou W, Yang J, Jia W, Xu Y. Wu L, et al. Front Oncol. 2023 Feb 7;13:1083085. doi: 10.3389/fonc.2023.1083085. eCollection 2023. Front Oncol. 2023. PMID: 36824145 Free PMC article. - Considering the Experimental use of Temozolomide in Glioblastoma Research.
Herbener VJ, Burster T, Goreth A, Pruss M, von Bandemer H, Baisch T, Fitzel R, Siegelin MD, Karpel-Massler G, Debatin KM, Westhoff MA, Strobel H. Herbener VJ, et al. Biomedicines. 2020 Jun 4;8(6):151. doi: 10.3390/biomedicines8060151. Biomedicines. 2020. PMID: 32512726 Free PMC article. Review. - Secondary anaplastic oligodendroglioma after cranial irradiation: a case report.
Doskaliyev A, Yamasaki F, Kenjo M, Shrestha P, Saito T, Hanaya R, Sugiyama K, Kurisu K. Doskaliyev A, et al. J Neurooncol. 2008 Jul;88(3):299-303. doi: 10.1007/s11060-008-9564-y. Epub 2008 Mar 29. J Neurooncol. 2008. PMID: 18373067
References
- Bower M, Newlands ES, Bleehen NM, Brada M, Begent RJ, Calvert H, Colquhoun I, Lewis P, Brampton MH (1997) Multicentre CRC phase II trial of temozolomide in recurrent or progressive high-grade glioma. Cancer Chemother Pharmacol 40: 484–488 - PubMed
- Brock CS, Newlands ES, Wedge SR, Bower M, Evans H, Colquhoun I, Roddie M, Glaser M, Brampton MH, Rustin GJ (1998) Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res 58: 4363–4367 - PubMed
- Denis LJ, Tolcher A, Figueroa JA, Drengler R, Geyer C, Eckhardt SG, Cutler Dl, Reyderman L, Von Hoff DD, Rowinsky EK (2000) Protracted daily administration of temozolomide is feasible: a phase I and pharmacokinetic – pharmacodynamic study. Proc Am Soc Clin Oncol 19: 202a
- Dolan ME, Moschel RC, Pegg AE (1990) Depletion of mammalian O6-alkylguanine-DNA alkyltransferase activity by O6-benzylguanine provides a means to evaluate the role of this protein in protection against carcinogenic and therapeutic alkylating agents. Proc Nat Acad Sci USA 87: 5368–5372 - PMC - PubMed
- Dumenco LL, Warman B, Hatzoglou M, Lim IK, Abboud SL, Gerson SL (1989) Increase in nitrosourea resistance in mammalian cells by retrovirally mediated gene transfer of bacterial O6-alkylguanine-DNA alkyltransferase. Cancer Res 49: 6044–6051 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials