Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease - PubMed (original) (raw)
Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease
Fangming Lin et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Polycystic kidney disease (PKD) is the most common genetic cause of renal failure in humans. Several proteins that are encoded by genes associated with PKD have recently been identified in primary cilia in renal tubular epithelia. These findings have suggested that abnormalities in cilia formation and function may play a role in the pathogenesis of PKD. To directly determine whether cilia are essential to maintain tubular integrity, we conditionally inactivated KIF3A, a subunit of kinesin-II that is essential for cilia formation, in renal epithelia. Constitutive inactivation of KIF3A produces abnormalities of left-right axis determination and embryonic lethality. Here we show that tissue-specific inactivation of KIF3A in renal tubular epithelial cells results in viable offspring with normal-appearing kidneys at birth. Cysts begin to develop in the kidney at postnatal day 5 and cause renal failure by postnatal day 21. The cyst epithelial cells lack primary cilia and exhibit increased proliferation and apoptosis, apical mislocalization of the epidermal growth factor receptor, increased expression of beta-catenin and c-Myc, and inhibition of p21(CIP1). These results demonstrate that the absence of renal cilia produces both the clinical and cell biological findings associated with PKD. Most generally, the phenotype of Kif3a mutant mice suggests a role for primary cilia in the maintenance of lumen-forming epithelial differentiation.
Figures
Figure 1
Kidney-specific inactivation of the Kif3a gene. (A) Map of the _Kif3a_fl allele before and after recombination. Primers P1 and P3 used for genotyping have been described (22). The sequence of primer P2′ was GGCAGATGGATCTCTGTGAGTTTG. (B) PCR products obtained after amplification of DNA from tails (T) of mice with the genotypes indicated and the kidneys (K) and brains (B) from _Kif3a_fl/+ mice and _cre;Kif3a_fl/+ littermates. Arrow indicates the band corresponding to the recombined (null) allele. (C) RT-PCR analysis of Kif3a and Gapdh mRNA expression in kidney (K) and brain (B) from Kif3a mutants (lanes 3 and 4) and controls (lanes 1 and 2 and 5 and 6) at P28. (D) Northern blot analysis of RNA from mutant kidneys (right lane) and control kidneys (left lane) at P28. (E) Immunoblot analysis of kidney extracts from Kif3a mutants (lanes 1, 2, and 4) and controls (lanes 3, 5, and 6) at P28.
Figure 2
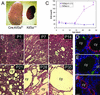
PKD in Kif3a mutant mice. (A) Gross appearance of kidneys from a Kif3a mutant (left) and a wild-type littermate (right) at P28. (B) Hematoxylin/eosin-stained sections of kidneys from Kif3a mutants at different ages. (C) Blood urea nitrogen of mutant mice (red) and control littermates (blue). Error bars = SEM. *, statistically different from control (P < 0.001, Student's t test). (D_–_F) Staining of cystic kidneys at P21 for AQP2 (green) and Tamm–Horsfall protein (red) (D), Lotus tetragonolobus agglutinin (green) and Na-K-Cl cotransporter (red) (E), and Na-Cl cotransporter (red) (F). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (blue). cy, cyst; pt, proximal tubule. [Bars = 1 mm (A), 80 μm (B), and 30 μm (D_–_F).]
Figure 3
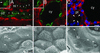
Absence of renal cilia in Kif3a mutant mice. (A_–_C) Immunostaining of acetylated tubulin (red) and AQP2 (green) in kidneys from control mice (A) and Kif3a mutants (B and C) at P21. Arrowheads, primary cilia; cd, collecting duct; hl, loop of Henle; pt, proximal tubule; cy, cyst. Insets show higher-magnification images. (Bar = 10 μm.) (D_–_F) Scanning electron micrographs of kidneys from control mice (D) and Kif3a mutants (E and F) at P21. Arrowheads, primary cilia; pc, principal cell; ic, intercalated cell; tu, tubule. [Bars = 10 (A_–_C) and 5 μm (D_–_F).]
Figure 4
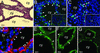
Abnormalities of proliferation, apoptosis, and cell polarity in Kif3a mutant mice. (A) Hematoxylin/eosin-stained kidney from a Kif3a mutant at P35. cy, cyst. (B) BrdUrd incorporation (arrowheads) in kidneys from a Kif3a mutant and control littermate (Inset) at P28. (C) Apoptotic cells (arrowheads) in kidneys from a Kif3a mutant and control littermate (Inset) at P21. (D) Localization of EGFR (red) and ZO-1 (green) in kidneys from a Kif3a mutant at P21. Arrowheads, colocalization in apical membrane; arrows, basolateral membrane; tu, tubule. (E) Expression of AQP3 in a cystic kidney at P28. Arrowheads, basolateral membrane. (F and G) Expression of AQP2 in cystic kidneys before (F) and 30 min after (G) treatment with vasopressin. Arrowheads, cytoplasmic vesicles; arrows, apical membrane. [Bars = 20 (A) and 10 μm (B_–_G).]
Figure 5
Abnormal expression of β-catenin, c-Myc, and p21CIP1 in Kif3a mutants. (A) Immunoblot analysis of kidney extracts from Kif3a mutants (lanes 2 and 4) and control littermates (lanes 1 and 3) at P21. (B and E) Expression of β-catenin in control (B) and cystic (E) kidneys at P21. Arrowheads, basolateral membrane; arrows, cytosol. (C and F) Expression of p21CIP1 (red) in control (C) and cystic (F) kidneys at P21. Arrowheads, expression in nuclei that were counterstained with 4′,6-diamidino-2-phenylindole (blue). (D and G) Expression of p16INK4a (red) in control (D) and cystic (G) kidneys at P21. tu, tubule; cy, cyst. (Bars = 5 μm.)
Comment in
- New insights into ciliary function: kidney cysts and photoreceptors.
Calvet JP. Calvet JP. Proc Natl Acad Sci U S A. 2003 May 13;100(10):5583-5. doi: 10.1073/pnas.1031799100. Epub 2003 May 5. Proc Natl Acad Sci U S A. 2003. PMID: 12732727 Free PMC article. No abstract available.
Similar articles
- Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia.
Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Patel V, et al. Hum Mol Genet. 2008 Jun 1;17(11):1578-90. doi: 10.1093/hmg/ddn045. Epub 2008 Feb 9. Hum Mol Genet. 2008. PMID: 18263895 Free PMC article. - Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis.
Cano DA, Sekine S, Hebrok M. Cano DA, et al. Gastroenterology. 2006 Dec;131(6):1856-69. doi: 10.1053/j.gastro.2006.10.050. Epub 2006 Oct 26. Gastroenterology. 2006. PMID: 17123526 - Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization.
Cano DA, Murcia NS, Pazour GJ, Hebrok M. Cano DA, et al. Development. 2004 Jul;131(14):3457-67. doi: 10.1242/dev.01189. Development. 2004. PMID: 15226261 - Apico-basal polarity in polycystic kidney disease epithelia.
Wilson PD. Wilson PD. Biochim Biophys Acta. 2011 Oct;1812(10):1239-48. doi: 10.1016/j.bbadis.2011.05.008. Epub 2011 Jun 1. Biochim Biophys Acta. 2011. PMID: 21658447 Review. - Polycystic kidney disease and the renal cilium.
Deane JA, Ricardo SD. Deane JA, et al. Nephrology (Carlton). 2007 Dec;12(6):559-64. doi: 10.1111/j.1440-1797.2007.00869.x. Nephrology (Carlton). 2007. PMID: 17995581 Review.
Cited by
- Who drives the ciliary highway?
Malicki J. Malicki J. Bioarchitecture. 2012 Jul-Aug;2(4):111-7. doi: 10.4161/bioa.21101. Epub 2012 Jul 1. Bioarchitecture. 2012. PMID: 22960672 Free PMC article. Review. - Genes Regulating Spermatogenesis and Sperm Function Associated With Rare Disorders.
Linn E, Ghanem L, Bhakta H, Greer C, Avella M. Linn E, et al. Front Cell Dev Biol. 2021 Feb 16;9:634536. doi: 10.3389/fcell.2021.634536. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33665191 Free PMC article. Review. - Kif3a controls murine nephron number via GLI3 repressor, cell survival, and gene expression in a lineage-specific manner.
Chi L, Galtseva A, Chen L, Mo R, Hui CC, Rosenblum ND. Chi L, et al. PLoS One. 2013 Jun 7;8(6):e65448. doi: 10.1371/journal.pone.0065448. Print 2013. PLoS One. 2013. PMID: 23762375 Free PMC article. - Cdc42 deficiency causes ciliary abnormalities and cystic kidneys.
Choi SY, Chacon-Heszele MF, Huang L, McKenna S, Wilson FP, Zuo X, Lipschutz JH. Choi SY, et al. J Am Soc Nephrol. 2013 Sep;24(9):1435-50. doi: 10.1681/ASN.2012121236. Epub 2013 Jun 13. J Am Soc Nephrol. 2013. PMID: 23766535 Free PMC article. - Disease-associated KIF3A variants alter gene methylation and expression impacting skin barrier and atopic dermatitis risk.
Stevens ML, Zhang Z, Johansson E, Ray S, Jagpal A, Ruff BP, Kothari A, He H, Martin LJ, Ji H, Wikenheiser-Brokamp K, Weirauch MT, Supp DM, Biagini Myers JM, Khurana Hershey GK. Stevens ML, et al. Nat Commun. 2020 Aug 14;11(1):4092. doi: 10.1038/s41467-020-17895-x. Nat Commun. 2020. PMID: 32796837 Free PMC article.
References
- Igarashi P, Somlo S. J Am Soc Nephrol. 2002;13:2384–2398. - PubMed
- Murcia N S, Sweeney W E, Jr, Avner E D. Kidney Int. 1999;55:1187–1197. - PubMed
- Harris P C. Curr Opin Nephrol Hypertens. 2002;11:309–314. - PubMed
- Wheatley D N. Pathobiology. 1995;63:222–238. - PubMed
- Praetorius H A, Spring K R. J Membr Biol. 2001;184:71–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials