In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation - PubMed (original) (raw)
In vitro and in vivo synergistic interactions between the Runx2/Cbfa1 transcription factor and bone morphogenetic protein-2 in stimulating osteoblast differentiation
Shuying Yang et al. J Bone Miner Res. 2003 Apr.
Abstract
Bone regeneration requires interactions between a number of factors including bone morphogenetic proteins (BMPs), growth factors, and transcriptional regulators such as Runx2/Cbfal (Runx2). Because each component may provide a unique contribution to the overall osteogenic response, we hypothesized that bone formation may be enhanced by using combinations of complimentary factors. As an initial test of this concept, interactions between BMP2 and Runx2 were examined using adenovirus-based expression vectors (AdCMV-Runx2, AdCMV-BMP2) in the pluripotent C3H10T1/2 cell line. Cells transduced with AdCMV-Runx2 strongly expressed osteoblast markers, such as alkaline phosphatase and osteocalcin, but formed only a weakly mineralized extracellular matrix in vitro, whereas cells transduced with AdCMV-BMP2 exhibited higher levels of mineralization, but only expressed low levels of Runx2 and osteocalcin mRNA. Significantly, when cells were transduced with optimal titers of both viruses, osteoblast differentiation was stimulated to levels that were 10-fold greater than those seen with either AdCMV-Runx2 or AdCMV-BMP2 alone. To measure in vivo osteogenic activity, virally transduced cells were subcutaneously implanted into immunodeficient mice. Cells transduced with control virus produced only fibrous tissue while those with AdCMV-Runx2 produced limited amounts of both cartilage and bone. In contrast, cells transduced with either AdCMV-BMP2 alone or AdCMV-BMP2 plus AdCMV-Cbfal generated large ossicles containing cartilage, bone, and a marrow cavity. However, ossification in the AdCMV-BMP2 plus AdCMV-Cbfal group was more extensive in that both mineral content and fractional bone area were greater than that seen in the AdCMV-BMP2 group. Thus, the increased osteoblast differentiation observed with combined adenovirus treatment in vitro is also manifested by increased bone formation in vivo. These results suggest that Runx2 and BMP2 have distinct, but complementary, roles in osteogenesis and that their combined actions may be necessary for optimal bone formation.
Figures
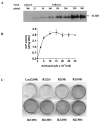
FIG. 1
Effect of adenovirus titer on Runx2 expression and induction of osteoblast differentiation in C3H10T1/2 cells. C3H10T1/2 cells were transduced with the indicated titer of AdCMV-LacZ (AdLacZ) or AdCMV-Runx2 (AdRunx2) as described in the Materials and Methods section. After 24 h, cells were fed with complete medium containing 10% FBS, 50 _μ_g/ml ascorbic acid, and 5 mM _β_-glycerol phosphate. (A) On day 3 after viral transduction, Runx2 protein levels were measured in whole cell extracts by Western blotting. (B) On day 6, cells were harvested for measurement of ALP activity. (C) A separate set of cultures was stained for mineralization by method of von Kossa after 12 days in culture.
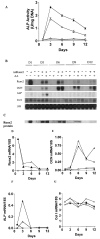
FIG. 2
Time course of osteoblast differentiation in AdRunx2-transduced C3H10T1/2 cells. Cultures were transduced with AdLacZ (~) or AdRunx2 (r,p) at a titer of 250 pfu/cell. After 24 h, one-half the cells in each group were fed with basal medium (_α_-MEM, 10% FBS, open symbols) and one-half were fed with basal medium supplemented with 50 _μ_g/ml ascorbic acid (closed symbols). Cells were harvested at the times indicated and assayed for (A) ALP activity, (B) mRNA levels, or (C) Runx2 protein levels. (D–G) These panels show mRNA levels after imaging and normalization to 18S rRNA.
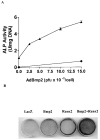
FIG. 3
Effect of Runx2 expression on AdBMP2-dependent induction of ALP and mineralization. Cells were transduced with the indicated titer of AdCMV-BMP2 in the presence (▴) or absence (●) of AdRunx2 at a titer of 100 pfu/cell. Total viral titer was held constant at 250 pfu/cell by addition of the appropriate titer of AdCMV-lac Z control virus. The cells were harvested (A) at day 6 for ALP assays or (B) at day 9 for measurement of mineralization by von Kossa staining.
FIG. 4
Time course of osteoblast differentiation in C3H10T1/2 cells transduced with AdBMP2 in the presence or absence of AdRunx2. Cells were transduced with the following combinations of adenovirus vectors: AdLacZ alone (200 pfu/cell), ●; AdLacZ (100 pfu/cell) plus AdBMP2 (100 pfu/cell), ●; AdLacZ (100 pfu/cell) plus AdRunx2 (100 pfu/cell), ▵; and AdBMP2 (100 pfu/cell) plus AdRunx2 (100 pfu/cell), ▴. Cells were harvested at the times indicated for measurement of (A) ALP activity, (B) calcium, (C) osteoblast marker mRNA expression, or (D) total Runx2 or BMP2 protein. Normalized mRNA levels are shown for (E) Runx2 mRNA and (F) OCN mRNA. (G) Replicate plates of cells were also stained for mineral by the method of von Kossa.
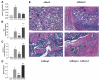
FIG. 5
In vivo bone formation by virally-transduced C3H10T1/2 cells. Cells were transduced with indicated adenoviruses and implanted into immunodeficient mice as described in Methods. After 4 weeks, transplants were harvested for determination of (A) total wet weigh, (B) ALP activity, or (C) calcium. (D) A morphometric analysis of histological sections from each treatment group. Results are expressed as the ratio of total bone area/total implant area. Statistical analysis: a, significantly different from AdLacZ (p < 0.001); b, significantly different from AdBMP2 (p < 0.05). (E) Histological sections of each treatment group. Implants of cells transduced with control virus contained residual Gelfoam carrier (g) and fibrous tissue (f), but no bone or cartilage. Cells transduced with AdRunx2 alone contained small areas of both bone (b) and cartilage (c) as well as a small marrow cavity (m). Both BMP2 alone and BMP2 plus Runx2 treated groups formed large ossicles with clearly defined cortical and trabecular bone as well as a marrow cavity.
Similar articles
- SWI/SNF chromatin remodeling complex is obligatory for BMP2-induced, Runx2-dependent skeletal gene expression that controls osteoblast differentiation.
Young DW, Pratap J, Javed A, Weiner B, Ohkawa Y, van Wijnen A, Montecino M, Stein GS, Stein JL, Imbalzano AN, Lian JB. Young DW, et al. J Cell Biochem. 2005 Mar 1;94(4):720-30. doi: 10.1002/jcb.20332. J Cell Biochem. 2005. PMID: 15565649 - BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype.
Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. Phimphilai M, et al. J Bone Miner Res. 2006 Apr;21(4):637-46. doi: 10.1359/jbmr.060109. Epub 2006 Apr 5. J Bone Miner Res. 2006. PMID: 16598384 Free PMC article. - Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts.
Cowan CM, Jiang X, Hsu T, Soo C, Zhang B, Wang JZ, Kuroda S, Wu B, Zhang Z, Zhang X, Ting K. Cowan CM, et al. J Bone Miner Res. 2007 Jun;22(6):918-30. doi: 10.1359/jbmr.070312. J Bone Miner Res. 2007. PMID: 17352654 Free PMC article. - The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration.
Zhang X, Zara J, Siu RK, Ting K, Soo C. Zhang X, et al. J Dent Res. 2010 Sep;89(9):865-78. doi: 10.1177/0022034510376401. Epub 2010 Jul 20. J Dent Res. 2010. PMID: 20647499 Free PMC article. Review. - [Research progress in osteogenesis and osteogenic mechanism of heparan sulfate].
Xu Z, Chen J, Shao W, Wang R, Liu Y. Xu Z, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017 Aug 15;31(8):1016-1020. doi: 10.7507/1002-1892.201701103. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017. PMID: 29806444 Free PMC article. Review. Chinese.
Cited by
- Gene therapy approaches for bone regeneration.
Franceschi RT, Yang S, Rutherford RB, Krebsbach PH, Zhao M, Wang D. Franceschi RT, et al. Cells Tissues Organs. 2004;176(1-3):95-108. doi: 10.1159/000075031. Cells Tissues Organs. 2004. PMID: 14745239 Free PMC article. Review. - Engineering graded tissue interfaces.
Phillips JE, Burns KL, Le Doux JM, Guldberg RE, García AJ. Phillips JE, et al. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12170-5. doi: 10.1073/pnas.0801988105. Epub 2008 Aug 21. Proc Natl Acad Sci U S A. 2008. PMID: 18719120 Free PMC article. - Oestrogen Inhibits Arterial Calcification by Promoting Autophagy.
Peng YQ, Xiong D, Lin X, Cui RR, Xu F, Zhong JY, Zhu T, Wu F, Mao MZ, Liao XB, Yuan LQ. Peng YQ, et al. Sci Rep. 2017 Jun 14;7(1):3549. doi: 10.1038/s41598-017-03801-x. Sci Rep. 2017. PMID: 28615727 Free PMC article. - Artificial Bone via Bone Tissue Engineering: Current Scenario and Challenges.
Kashte S, Jaiswal AK, Kadam S. Kashte S, et al. Tissue Eng Regen Med. 2017 Jan 17;14(1):1-14. doi: 10.1007/s13770-016-0001-6. eCollection 2017 Feb. Tissue Eng Regen Med. 2017. PMID: 30603457 Free PMC article. Review. - Effect of Total Flavonoids of Rhizoma drynariae on Tibial Dyschondroplasia by Regulating BMP-2 and Runx2 Expression in Chickens.
Yao W, Zhang H, Jiang X, Mehmood K, Iqbal M, Li A, Zhang J, Wang Y, Waqas M, Shen Y, Li J. Yao W, et al. Front Pharmacol. 2018 Nov 2;9:1251. doi: 10.3389/fphar.2018.01251. eCollection 2018. Front Pharmacol. 2018. PMID: 30450047 Free PMC article.
References
- Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393–411. - PubMed
- Lian JB, Stein GS. Osteoblast biology. In: Marcus R, Feldman D, Kelsey J, editors. Osteoporosis. 2nd ed. Vol. 1. Academic Press; San Diego, CA, USA: 2001. pp. 21–71.
- Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81:710–718. - PubMed
- Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: In vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000;78:476–486. - PubMed
- Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201–1210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DE13386/DE/NIDCR NIH HHS/United States
- R01 DE013386/DE/NIDCR NIH HHS/United States
- R01 DE011723/DE/NIDCR NIH HHS/United States
- DE11723/DE/NIDCR NIH HHS/United States
- DE13835/DE/NIDCR NIH HHS/United States
- R56 DE011723/DE/NIDCR NIH HHS/United States
- R01 DE013835/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials