Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice - PubMed (original) (raw)
Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice
Masahiro Fukaya et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Glutamate is a major excitatory neurotransmitter in the mammalian central nervous system, and the N-methyl-D-aspartate-selective glutamate receptor (NR) consisting of the NR1 subunit and an NR2 or NR3 subunit plays crucial roles in synaptic transmission, plasticity, and learning and memory. By using a knockout mouse strain, in which the NR1 gene deletion is primarily targeted to the CA1 pyramidal cells of the hippocampus, we investigated the in vivo effect of the loss of the NR1 subunit on the cellular expression and intracellular distribution of the NR2 subunits. The NR1 gene deletion had no apparent effect on the levels of NR2A or NR2B mRNA but led to severe reductions of NR2A and NR2B protein in dendrites of CA1 pyramidal cells. This reduced dendritic distribution of the NR2 subunits accompanied their robust accumulation in perikarya, where they were condensed in the lumen of the endoplasmic reticulum as electron-dense granules. These granules were also observed in CA1 pyramidal cells of the control mice but they were much fewer and contained no detectable levels of the NR2 subunit. The effect of the NR1 knockout on intracellular localization of the NR2 subunits was specific in that no such effect was observed for the GluR1 and PSD-95, two other major postsynaptic proteins. These results suggest that the NR1 subunit plays a crucial role in the release of the NR2 subunit from the endoplasmic reticulum in hippocampal pyramidal cells in vivo, and when the NR1 subunit is unavailable, the NR2 subunits are retained and aggregate into intracisternal granules.
Figures
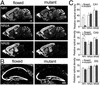
Figure 1
Age-dependent NR1 knockout in the CA1-NR1 knockout mouse. (A) In situ hybridization with antisense NR1 probe. Photographs are negative images printed directly from x-ray films. (B) Immunoperoxidase with NR1 Ab. Pairs of floxed and mutant brains were embedded in single paraffin blocks to be processed simultaneously. Arrows and arrowheads indicate the hippocampal CA1 region at stages when NR1 reduction is first observed or reaches the lowest level, respectively. 1M–4M, 1–4 months of age; Cb, cerebellum; CP, caudate-putamen; Cx, cortex; Hi, hippocampus; Th, thalamus. (Bars = 1 mm.)
Figure 2
Transcription levels of NR1, NR2A, and NR2B mRNAs in the hippocampus at 2 months of age. (A) X-ray film autoradiography for NR1 (Top), NR2A (Middle), and NR2B (Bottom) mRNAs using adjacent parasagittal brain sections. Note normal expressions of NR2A and NR2B mRNAs in the mutant CA1 region, in contrast to virtual disappearance of NR1 mRNA (white arrowhead). (B) Emulsion-dipped microautoradiography for NR1 mRNA. Arrows indicate a slight reduction of NR1 mRNA in the CA3 region. (C) Semiquantification of x-ray film autoradiograms to compare transcription levels in the CA1 region (Top), CA3 region (Middle), and dentate gyrus (DG, Bottom). The mean and SDs were calculated from three floxed or three mutant mice. 1–3, CA1–CA3 regions. *, P < 0.001; **, P < 0.01. [Bars = 1 mm (A) and 0.1 mm (B).]
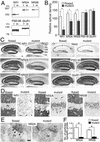
Figure 3
Immunoblot and immunohistochemistry for NR1, NR2A, NR2B, PSD-95, and GluR1 in the hippocampus at 2 months of age. (A) Immunoblot using the whole hippocampal protein extracts from floxed (f) and mutant (m) mice. (B) The relative optical density of immunoblot protein bands. Significant reductions are observed for NR1, NR2A, and NR2B subunits (*, P < 0.05 for each). Similar results were obtained with x-ray films of different exposure times, indicating the detection within the linear range of signal intensity. (C) Histology and immunohistochemistry. (D) High-power views of immunostained CA1 region. Note a dense accumulation of NR2A and NR2B subunits in perikarya of mutant pyramidal cells, in contrast to almost negative labeling in those of the floxed cells. Arrows indicate immunopositive dendritic shafts of putative interneurons. (E) Postembedding immunogold staining for NR2A subunit at asymmetrical axo-spinous synapses of floxed and mutant CA1 region. Note the lack of gold labeling in the mutant synapse, in contrast to postsynaptic labeling in the floxed one (arrowheads). (F) The number of immunogold particles per profile of asymmetrical axo-spinous synapses in the CA1 region of the floxed (white bars) and mutant (black bars) mice. Immunogold labeling is significantly reduced for both NR2A and NR2B subunits (*, P < 0.01). Cx, cortex; DG, dentate gyrus; Gr, granule cell layer; LM, stratum lacunosum-moleculare; Mo, stratum moleculare; NT, nerve terminal; Or, stratum oriens; Py, pyramidal cell layer; Ra, stratum radiatum; Sp, spine; Su, subiculum; Th, thalamus; 1–3, CA1–CA3 regions. [Bars = 0.1 mm (C), 20 μm (D), and 100 nm (E).]
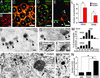
Figure 4
Immunofluorescence and electron microscopic analyses demonstrating ER retention of NR2 subunits in mutant CA1 pyramidal cells at 2 months of age. (A_–_D) Double immunofluorescence for NR2A (green) and ER marker calreticulin (Cal., red) or lysosomal marker cathepsin D (Cat-D, red) in mutant CA1 pyramidal cells. White asterisks indicate the nucleus of pyramidal cells. (E) Double immunofluorescence for NR2A (green) and Cal. (red) in a mutant CA1 interneuron (white arrow). NR2A subunit is detected as tiny puncta along the surface of the cell body and dendritic processes of this interneuron (white arrowheads). (F_–_I) Postembedding immunogold labeling for the NR2A (F and H) and NR2B (G and I) subunits in CA1 pyramidal cells of the mutant (F and G) and floxed (H and I) hippocampi. Black arrowheads and arrows indicate immunogold-positive or -negative granules in the ER, respectively. (J and K) Conventional electron microscopy showing the presence of electron-dense granules (black arrows) in the ER lumen of CA1 pyramidal cell perikarya in mutant (J) and floxed (K) mice. (L) The number of NR2A and NR2B immunogold particles per profile of ICGs in CA1 pyramidal cells of the floxed (blue bars) and mutant (red bars) mice (*, P < 0.01). (M) Histograms for the central-to-peripheral distribution of immunogold particles for the NR2A (Upper) and NR2B (Lower) subunits on ICG profiles in the mutant CA1 pyramidal cells. The scores of 0% and 100% indicate the center or edge of electron-dense ICGs, respectively, and the distance from the center of ICGs to the center of gold particles is plotted as 20% wide bins. (N) The number of ICGs per pyramidal cell profile on electron micrographs in the C57BL (wild), floxed, and mutant mice (*, P < 0.01). Go, Golgi apparatus; Ly, lysosome; Nu, nucleus. [Bars = 10 μm (A_–_E) and 100 nm (F_–_K).]
Similar articles
- Export from the endoplasmic reticulum of assembled N-methyl-d-aspartic acid receptors is controlled by a motif in the c terminus of the NR2 subunit.
Hawkins LM, Prybylowski K, Chang K, Moussan C, Stephenson FA, Wenthold RJ. Hawkins LM, et al. J Biol Chem. 2004 Jul 9;279(28):28903-10. doi: 10.1074/jbc.M402599200. Epub 2004 Apr 21. J Biol Chem. 2004. PMID: 15102836 - Formation of NR1/NR2 and NR1/NR3 heterodimers constitutes the initial step in N-methyl-D-aspartate receptor assembly.
Schüler T, Mesic I, Madry C, Bartholomäus I, Laube B. Schüler T, et al. J Biol Chem. 2008 Jan 4;283(1):37-46. doi: 10.1074/jbc.M703539200. Epub 2007 Oct 24. J Biol Chem. 2008. PMID: 17959602 - A three amino acid tail following the TM4 region of the N-methyl-D-aspartate receptor (NR) 2 subunits is sufficient to overcome endoplasmic reticulum retention of NR1-1a subunit.
Yang W, Zheng C, Song Q, Yang X, Qiu S, Liu C, Chen Z, Duan S, Luo J. Yang W, et al. J Biol Chem. 2007 Mar 23;282(12):9269-78. doi: 10.1074/jbc.M700050200. Epub 2007 Jan 25. J Biol Chem. 2007. PMID: 17255096 - New insights into the not-so-new NR3 subunits of N-methyl-D-aspartate receptor: localization, structure, and function.
Low CM, Wee KS. Low CM, et al. Mol Pharmacol. 2010 Jul;78(1):1-11. doi: 10.1124/mol.110.064006. Epub 2010 Apr 2. Mol Pharmacol. 2010. PMID: 20363861 Review. - 2-{[4-(4-[125I]Iodobenzyl)piperidin-1-yl]methyl}benzimidazol-5-ol.
Chopra A. Chopra A. 2011 Jan 31 [updated 2011 Mar 31]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Jan 31 [updated 2011 Mar 31]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21473031 Free Books & Documents. Review.
Cited by
- Minocycline reduces neuroinflammation but does not ameliorate neuron loss in a mouse model of neurodegeneration.
Cheng S, Hou J, Zhang C, Xu C, Wang L, Zou X, Yu H, Shi Y, Yin Z, Chen G. Cheng S, et al. Sci Rep. 2015 May 22;5:10535. doi: 10.1038/srep10535. Sci Rep. 2015. PMID: 26000566 Free PMC article. - Synaptic and cellular changes induced by the schizophrenia susceptibility gene G72 are rescued by N-acetylcysteine treatment.
Pósfai B, Cserép C, Hegedüs P, Szabadits E, Otte DM, Zimmer A, Watanabe M, Freund TF, Nyiri G. Pósfai B, et al. Transl Psychiatry. 2016 May 10;6(5):e807. doi: 10.1038/tp.2016.74. Transl Psychiatry. 2016. PMID: 27163208 Free PMC article. - Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity.
Son GH, Geum D, Chung S, Kim EJ, Jo JH, Kim CM, Lee KH, Kim H, Choi S, Kim HT, Lee CJ, Kim K. Son GH, et al. J Neurosci. 2006 Mar 22;26(12):3309-18. doi: 10.1523/JNEUROSCI.3850-05.2006. J Neurosci. 2006. PMID: 16554481 Free PMC article. - Heterozygous deletion of NR1 subunit of the NMDA receptor alters ethanol-related behaviors and regional expression of NR2 subunits in the brain.
Du X, Elberger AJ, Matthews DB, Hamre KM. Du X, et al. Neurotoxicol Teratol. 2012 Jan-Feb;34(1):177-86. doi: 10.1016/j.ntt.2011.09.001. Epub 2011 Sep 14. Neurotoxicol Teratol. 2012. PMID: 21945132 Free PMC article. - Silencing CA1 pyramidal cells output reveals the role of feedback inhibition in hippocampal oscillations.
Adaikkan C, Joseph J, Foustoukos G, Wang J, Polygalov D, Boehringer R, Middleton SJ, Huang AJY, Tsai LH, McHugh TJ. Adaikkan C, et al. Nat Commun. 2024 Mar 11;15(1):2190. doi: 10.1038/s41467-024-46478-3. Nat Commun. 2024. PMID: 38467602 Free PMC article.
References
- Bliss T V, Collingridge G L. Nature. 1993;361:31–39. - PubMed
- Seeburg P H. Trends Neurosci. 1993;16:359–365. - PubMed
- Nakanishi S, Masu M. Annu Rev Biophys Biomol Struct. 1994;23:319–348. - PubMed
- Mori H, Mishina M. Neuropharmacology. 1995;34:1219–1237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous