Circadian and photic regulation of phosphorylation of ERK1/2 and Elk-1 in the suprachiasmatic nuclei of the Syrian hamster - PubMed (original) (raw)
Circadian and photic regulation of phosphorylation of ERK1/2 and Elk-1 in the suprachiasmatic nuclei of the Syrian hamster
Andrew N Coogan et al. J Neurosci. 2003.
Abstract
In this study we investigated the circadian and photic regulation of phosphorylation of the extracellular signal-related kinase (ERK) 1/2, and the transcription factor Elk-1 in the suprachiasmatic nuclei of the Syrian hamster. We report that levels of phosphorylated ERK (P-ERK) are rhythmic, peaking during the mid subjective day, whereas phosphorylated Elk-1 (P-Elk-1) shows no distinct rhythm. Light pulses during the subjective night rapidly, but transiently, induce P-ERK, whereas P-Elk-1 is also induced, albeit with a slower time course. Application of the ERK pathway inhibitor U0126 attenuates photic induction of both P-ERK and P-Elk-1 and phase advances of wheel-running behavior. The NMDA receptor channel blocker, MK-801, also significantly attenuates photic induction of P-ERK and P-Elk-1. Taken together, these results indicate a role of the ERK cascade in the regulation of free-running circadian rhythms and of photic-resetting of these rhythms and suggest that in the mammalian suprachiasmatic nuclei, Elk-1 represents a novel molecular component of the photic-induction pathway.
Figures
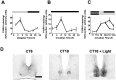
Fig. 1.
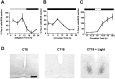
Regulation of P-ERK in the hamster SCN.A, Diurnal variation of P-ERK levels across an LD cycle.B, Circadian variation of P-ERK in constant darkness. Under both diurnal and free-running conditions, P-ERK levels peak during the mid subjective day, and levels are low throughout the subjective night. C, Photic regulation of P-ERK in the hamster SCN. Animals were sampled before, during, and after a 30 min light pulse (CT18–18.5; solid line) and during a 90 min (CT18–19.5) light pulse (dashed line). Onset of the light pulse triggered a large rise in levels of P-ERK in the SCN that rapidly declined toward baseline on termination of the pulse. Elongation of the light pulse led to sustained P-ERK levels.n = 4–6 for each point in_A_–C. D, Representative photomicrographs of P-ERK staining in the SCN sampled during the subjective day in DD (CT8), during the subjective night in DD (CT18), and 30 min into a light pulse (CT18 + Light). Scale bar, 50 μm.
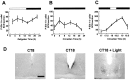
Fig. 2.
Regulation of P-Elk-1 in the hamster SCN.A, Diurnal variation of P-Elk-1 levels across the LD cycle. B, Circadian variation of P-Elk-1 in constant darkness. Under both diurnal and free-running conditions, P-Elk-1 levels did not vary significantly. C, Photic regulation of P-Elk-1 in the hamster SCN. Animals were sampled before, during, and after a 30 min light pulse (CT18–18.5; solid line). Application of the light pulse triggered a rise in levels of P-Elk-1 in the SCN, with peak levels achieved 60 min after onset of the pulse.n = 4–6 for each point in_A_–C. D, Representative photomicrographs of P-Elk-1 staining in the SCN sampled during the subjective day in DD (CT8), during the subjective night in DD (CT18), and 60 min after onset of a light pulse (CT18 + Light). Scale bar, 50 μm.
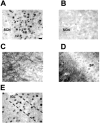
Fig. 3.
P-Elk-1 staining in other areas of the hamster brain. A, P-Elk-1 staining in the SCN was always found to be nuclear, with no discernable dendritic or axonal staining present (section from CT19, light pulse given CT18–18.5).B, Adjacent section to that shown in A, but primary antiserum was adsorbed with phosphorylated Elk-1 peptide. This treatment abolishes P-Elk-1 staining. C, P-Elk-1 staining in the hamster parietal cortex. Here the P-Elk-1 staining is seen to be mostly dendritic and axonal in character, with only faintly stained nuclei present, most notably in layers I, II, and III.D, P-Elk-1 staining in hippocampal CA1. Similar to the cortex, P-Elk-1 staining in the hippocampus is dendritic and axonal in character, present most notably in the stratum radiatum (SR). Weakly stained nuclei are present in the stratum pyramidale (SP).E, P-Elk-1 in the intergeniculate leaflet (IGL). The IGL is part of the geniculate complex and is known to play a part in circadian time keeping in mammals. P-Elk-1-ir was present in the nuclei of IGL cells. P-Elk-1 levels in the IGL were not affected by photic conditions or by circadian phase. Scale bar: (in A)A_–_E, 15 μm.
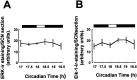
Fig. 4.
Total levels of ERK and Elk-1 do not vary in the SCN. Total levels of ERK (A) and Elk-1 (B), regardless of phosphorylation state, were not altered by light pulses at CT18. n = 4–6 for each point in A and B. ERK and Elk-1 also did not vary across the circadian or diurnal cycle (data not shown).
Fig. 5.
Regulation of c-Fos in the hamster SCN. Diurnal (A) and circadian (B) variation of c-Fos in the hamster SCN. Numbers of c-Fos-ir cells peak during the mid subjective day, and levels are low throughout the subjective night.C, Photic regulation of c-Fos in the hamster SCN. Light pulses led to a large rise in the number of c-Fos-ir cells in the SCN.n = 4–6 for each point in_A_–C. D, Representative photomicrographs of c-Fos-ir in the SCN sampled during the subjective day in DD (CT8), during the subjective night in DD (CT18), and 90 min after the start of the light pulse (CT18 + Light). Scale bar, 50 μm.
Fig. 6.
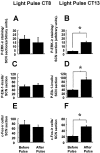
Phase specificity of photic induction of P-ERK, P-Elk-1, and c-Fos in the hamster SCN. Similar to a light pulse at CT18, a light pulse presented at CT13–13.5 (which leads to behavioral phase delays) upregulates P-ERK, P-Elk-1, and c-Fos in the hamster SCN (B, D, F). However, light pulse presented at CT8–CT8.5, which does not lead to behavioral phase shifts, also does not significantly alter levels of P-ERK, P-Elk-1, or c-Fos (A, C,D). n = 4 for both the prepulse and light-pulsed groups. *p < 0.01.
Fig. 7.
The NMDA-receptor channel blocker MK-801 attenuates photic induction of P-ERK (A, B), P-Elk-1 (C, D), and c-Fos (E, F) in the SCN. n = 4 for vehicle controls and MK-801 treatments. Representative photomicrographs of sections from CT18.5 (A), CT19 (C), and CT19.2 (E) after a light pulse at CT18. *p < 0.01. Scale bar, 50 μm.
Fig. 8.
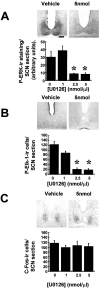
The ERK cascade inhibitor U0126 attenuates photically induced P-ERK and P-Elk-1 but not c-Fos in the SCN. Microinjection of 2.5 nmol (n = 5) or 5 nmol (n = 6) of U0126 (1 μl) into the third ventricle before application of a light pulse led to significant attenuation of P-ERK and P-Elk-1 induction (A, B) but did not attenuate c-Fos induction (C). Vehicle DMSO (1 μl/10%; n = 6) or 1 nmol of U0126 (n = 4) did not have any significant effect. *p < 0.01. Scale bar, 50 mm.
Fig. 9.
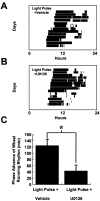
The ERK cascade inhibitor U0126 attenuates photically induced phase shifts of behavioral rhythms. Microinjection of U0126 (5 nmol in 1 μl; n = 6) (A) 30 min before a light pulse (CT18–18.5) significantly decreased the magnitude of phase advance compared with vehicle-treated controls (n = 5; B,C). In A and B, the light-filled circle denotes application of the light pulse, and in_C_ *p < 0.01.
Similar articles
- Dark pulse suppression of P-ERK and c-Fos in the hamster suprachiasmatic nuclei.
Coogan AN, Piggins HD. Coogan AN, et al. Eur J Neurosci. 2005 Jul;22(1):158-68. doi: 10.1111/j.1460-9568.2005.04193.x. Eur J Neurosci. 2005. PMID: 16029205 - Non-photic phase shifting of the circadian clock: role of the extracellular signal-responsive kinases I/II/mitogen-activated protein kinase pathway.
Antle MC, Tse F, Koke SJ, Sterniczuk R, Hagel K. Antle MC, et al. Eur J Neurosci. 2008 Dec;28(12):2511-8. doi: 10.1111/j.1460-9568.2008.06533.x. Eur J Neurosci. 2008. PMID: 19087176 - Nerve growth factor-induced circadian phase shifts and MAP kinase activation in the hamster suprachiasmatic nuclei.
Pizzio GA, Hainich EC, Plano SA, Ralph MR, Golombek DA. Pizzio GA, et al. Eur J Neurosci. 2005 Aug;22(3):665-71. doi: 10.1111/j.1460-9568.2005.04247.x. Eur J Neurosci. 2005. PMID: 16101748 - Convergence of the SUMO and MAPK pathways on the ETS-domain transcription factor Elk-1.
Yang SH, Sharrocks AD. Yang SH, et al. Biochem Soc Symp. 2006;(73):121-9. doi: 10.1042/bss0730121. Biochem Soc Symp. 2006. PMID: 16626293 Review. - SUMO and transcriptional repression: dynamic interactions between the MAP kinase and SUMO pathways.
Yang SH, Jaffray E, Senthinathan B, Hay RT, Sharrocks AD. Yang SH, et al. Cell Cycle. 2003 Nov-Dec;2(6):528-30. doi: 10.4161/cc.2.6.597. Cell Cycle. 2003. PMID: 14504467 Review.
Cited by
- Circadian Rhythm Protein Bmal1 Modulates Cartilage Gene Expression in Temporomandibular Joint Osteoarthritis via the MAPK/ERK Pathway.
Chen G, Zhao H, Ma S, Chen L, Wu G, Zhu Y, Zhu J, Ma C, Zhao H. Chen G, et al. Front Pharmacol. 2020 Sep 18;11:527744. doi: 10.3389/fphar.2020.527744. eCollection 2020. Front Pharmacol. 2020. PMID: 33041790 Free PMC article. - The suprachiasmatic nucleus is a functionally heterogeneous timekeeping organ.
Silver R, Schwartz WJ. Silver R, et al. Methods Enzymol. 2005;393:451-65. doi: 10.1016/S0076-6879(05)93022-X. Methods Enzymol. 2005. PMID: 15817305 Free PMC article. - The molecular gatekeeper Dexras1 sculpts the photic responsiveness of the mammalian circadian clock.
Cheng HY, Dziema H, Papp J, Mathur DP, Koletar M, Ralph MR, Penninger JM, Obrietan K. Cheng HY, et al. J Neurosci. 2006 Dec 13;26(50):12984-95. doi: 10.1523/JNEUROSCI.4253-06.2006. J Neurosci. 2006. PMID: 17167088 Free PMC article. - Autonomic deficit not the cause of death in West Nile virus neurological disease.
Wang H, Siddharthan V, Hall JO, Morrey JD. Wang H, et al. Clin Auton Res. 2014 Feb;24(1):15-23. doi: 10.1007/s10286-013-0213-y. Epub 2013 Oct 25. Clin Auton Res. 2014. PMID: 24158383 Free PMC article. - High-fat feeding alters the clock synchronization to light.
Mendoza J, Pévet P, Challet E. Mendoza J, et al. J Physiol. 2008 Dec 15;586(24):5901-10. doi: 10.1113/jphysiol.2008.159566. Epub 2008 Oct 20. J Physiol. 2008. PMID: 18936083 Free PMC article.
References
- Abe H, Rusak B, Robertson HA. Photic induction of Fos protein in the suprachiasmatic nucleus is inhibited by the NMDA receptor antagonist MK-801. Neurosci Lett. 1991;127:9–12. - PubMed
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. - PubMed
- Allada R, Emery P, Takahashi JS, Rosbash M. Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci. 2001;24:1091–1119. - PubMed
- Arima H, House SB, Gainer H, Aguilera G. Neuronal activity is required for the circadian rhythm of vasopressin gene transcription in the suprachiasmatic nucleus in vitro. Endocrinology. 2002;143:4165–4171. - PubMed
- Butcher G, Dziema H, Collamore M, Burgoon PW, Obrietan K. The p42/44 MAP kinase pathway couples photic input to circadian clock entrainment. J Biol Chem. 2002;277:29519–29525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous