Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent - PubMed (original) (raw)
Accumulation of cytoplasmic dynein and dynactin at microtubule plus ends in Aspergillus nidulans is kinesin dependent
Jun Zhang et al. Mol Biol Cell. 2003 Apr.
Abstract
The mechanism(s) by which microtubule plus-end tracking proteins are targeted is unknown. In the filamentous fungus Aspergillus nidulans, both cytoplasmic dynein and NUDF, the homolog of the LIS1 protein, localize to microtubule plus ends as comet-like structures. Herein, we show that NUDM, the p150 subunit of dynactin, also forms dynamic comet-like structures at microtubule plus ends. By examining proteins tagged with green fluorescent protein in different loss-of-function mutants, we demonstrate that dynactin and cytoplasmic dynein require each other for microtubule plus-end accumulation, and the presence of cytoplasmic dynein is also important for NUDF's plus-end accumulation. Interestingly, deletion of NUDF increases the overall accumulation of dynein and dynactin at plus ends, suggesting that NUDF may facilitate minus-end-directed dynein movement. Finally, we demonstrate that a conventional kinesin, KINA, is required for the microtubule plus-end accumulation of cytoplasmic dynein and dynactin, but not of NUDF.
Figures
Figure 1
(A) Dynamics of GFP-NUDM dynactin comets in vivo. Bar, ∼5 μm. (B) Immunostaining of GFP-NUDM and microtubules. This image resulted from a merge of a pseudocolored microtubule image (red) and a GFP-NUDM image (green).
Figure 2
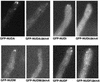
Images of GFP-NUDM dynactin in wild-type (GFP-NUDM) and the Δ_nudA_ mutant (GFP-NUDM/ΔnudA) cells, GFP-TUBA (α tubulin) in the Δ_nudA_ mutant (GFP-TUBA/ΔnudA), and GFP-NUDA dynein heavy chain in wild-type (GFP-NUDA) and the nudM116 mutant (GFP-NUDA/nudM116) cells.
Figure 3
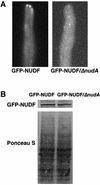
(A) Images of GFP-NUDF (LIS1-like protein) in wild-type and the Δ_nudA_ mutant cells. (B) Western blot analysis on the level of GFP-NUDF in protein extract from the wild-type and the Δ_nudA_ mutant cells. A monoclonal anti-GFP antibody (BD Biosciences Clontech) was used at 1/500. The level of protein loading was shown by Ponceau S staining of the membrane.
Figure 4
Images of GFP-NUDA (dynein HC), GFP-NUDI (dynein IC), and GFP-NUDM (p150 dynactin) in wild-type and the Δ_nudF_ mutant cells.
Figure 5
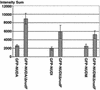
Graphic presentation of the fluorescence intensities of GFP comets near the hyphal tip. Values of mean and SD were generated based on 12 individual comets of each strain.
Figure 6
Images of GFP-NUDA (dynein HC), GFP-NUDI (dynein IC), GFP-NUDM (p150 dynactin), and GFP-NUDF (LIS1-like protein) in wild-type and the Δ_kinA_ mutant cells.
Figure 7
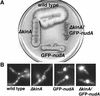
NudA/kinA double mutant analyses. (A) Growth of the wild-type, Δ_kinA_, GFP-nudA, and _ΔkinA/_GFP-nudA (JZ4) strains on YUU that represses the expression of the alcA_-driven GFP-nudA fusion gene, the only intact version of nudA in the genome. The plate was incubated at 32°C for 2 d. (B) 4,6-Diamidino-2-phenylindole staining of the wild-type, Δ_kinA, GFP-nudA, and _ΔkinA/_GFP-nudA (JZ4) cells that were grown in YUU at 32°C for 7.5 h.
Figure 8
Model providing an explanation for the observation that accumulation of dynein/dynactin at the microtubule plus ends is increased in the nudF deletion mutant. NUDF/LIS1 may facilitate dynein-mediated cargo departure from the plus end toward the minus end of the microtubule. Failure of such departure is expected to cause a more intense plus-end accumulation of dynein/dynactin, which is what has been found in absence of NUDF.
Similar articles
- CLIP-170 homologue and NUDE play overlapping roles in NUDF localization in Aspergillus nidulans.
Efimov VP, Zhang J, Xiang X. Efimov VP, et al. Mol Biol Cell. 2006 Apr;17(4):2021-34. doi: 10.1091/mbc.e05-11-1084. Epub 2006 Feb 8. Mol Biol Cell. 2006. PMID: 16467375 Free PMC article. - The Aspergillus cytoplasmic dynein heavy chain and NUDF localize to microtubule ends and affect microtubule dynamics.
Han G, Liu B, Zhang J, Zuo W, Morris NR, Xiang X. Han G, et al. Curr Biol. 2001 May 1;11(9):719-24. doi: 10.1016/s0960-9822(01)00200-7. Curr Biol. 2001. PMID: 11369237 - In vivo roles of the basic domain of dynactin p150 in microtubule plus-end tracking and dynein function.
Yao X, Zhang J, Zhou H, Wang E, Xiang X. Yao X, et al. Traffic. 2012 Mar;13(3):375-87. doi: 10.1111/j.1600-0854.2011.01312.x. Epub 2011 Dec 18. Traffic. 2012. PMID: 22106867 Free PMC article. - Cytoplasmic dynein and early endosome transport.
Xiang X, Qiu R, Yao X, Arst HN Jr, Peñalva MA, Zhang J. Xiang X, et al. Cell Mol Life Sci. 2015 Sep;72(17):3267-80. doi: 10.1007/s00018-015-1926-y. Epub 2015 May 23. Cell Mol Life Sci. 2015. PMID: 26001903 Free PMC article. Review. - Cytoplasmic dynein and dynactin in cell division and intracellular transport.
Karki S, Holzbaur EL. Karki S, et al. Curr Opin Cell Biol. 1999 Feb;11(1):45-53. doi: 10.1016/s0955-0674(99)80006-4. Curr Opin Cell Biol. 1999. PMID: 10047518 Review.
Cited by
- Kinesin Motors in the Filamentous Basidiomycetes in Light of the Schizophyllum commune Genome.
Raudaskoski M. Raudaskoski M. J Fungi (Basel). 2022 Mar 12;8(3):294. doi: 10.3390/jof8030294. J Fungi (Basel). 2022. PMID: 35330296 Free PMC article. Review. - Lissencephaly-1 is a context-dependent regulator of the human dynein complex.
Baumbach J, Murthy A, McClintock MA, Dix CI, Zalyte R, Hoang HT, Bullock SL. Baumbach J, et al. Elife. 2017 Apr 13;6:e21768. doi: 10.7554/eLife.21768. Elife. 2017. PMID: 28406398 Free PMC article. - BICD2, dynactin, and LIS1 cooperate in regulating dynein recruitment to cellular structures.
Splinter D, Razafsky DS, Schlager MA, Serra-Marques A, Grigoriev I, Demmers J, Keijzer N, Jiang K, Poser I, Hyman AA, Hoogenraad CC, King SJ, Akhmanova A. Splinter D, et al. Mol Biol Cell. 2012 Nov;23(21):4226-41. doi: 10.1091/mbc.E12-03-0210. Epub 2012 Sep 5. Mol Biol Cell. 2012. PMID: 22956769 Free PMC article. - Aspergillus myosin-V supports polarized growth in the absence of microtubule-based transport.
Zhang J, Tan K, Wu X, Chen G, Sun J, Reck-Peterson SL, Hammer JA 3rd, Xiang X. Zhang J, et al. PLoS One. 2011;6(12):e28575. doi: 10.1371/journal.pone.0028575. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194856 Free PMC article. - CLIP-170 homologue and NUDE play overlapping roles in NUDF localization in Aspergillus nidulans.
Efimov VP, Zhang J, Xiang X. Efimov VP, et al. Mol Biol Cell. 2006 Apr;17(4):2021-34. doi: 10.1091/mbc.e05-11-1084. Epub 2006 Feb 8. Mol Biol Cell. 2006. PMID: 16467375 Free PMC article.
References
- Asai DJ, Koonce MP. The dynein heavy chain: structure, mechanics and evolution. Trends Cell Biol. 2001;11:196–202. - PubMed
- Brunner D, Nurse P. CLIP170-like tip1p spatially organizes microtubular dynamics in fission yeast. Cell. 2000;102:695–704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous