Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores - PubMed (original) (raw)
Human MPS1 kinase is required for mitotic arrest induced by the loss of CENP-E from kinetochores
Song-Tao Liu et al. Mol Biol Cell. 2003 Apr.
Abstract
We have determined that the previously identified dual-specificity protein kinase TTK is the human orthologue of the yeast MPS1 kinase. Yeast MPS1 (monopolar spindle) is required for spindle pole duplication and the spindle checkpoint. Consistent with the recently identified vertebrate MPS1 homologues, we found that hMPS1 is localized to centrosomes and kinetochores. In addition, hMPS1 is part of a growing list of kinetochore proteins that are localized to nuclear pores. hMPS1 is required by cells to arrest in mitosis in response to spindle defects and kinetochore defects resulting from the loss of the kinesin-like protein, CENP-E. The pattern of kinetochore localization of hMPS1 in CENP-E defective cells suggests that their interaction with the kinetochore is sensitive to microtubule occupancy rather than kinetochore tension. hMPS1 is required for MAD1, MAD2 but not hBUB1, hBUBR1 and hROD to bind to kinetochores. We localized the kinetochore targeting domain in hMPS1 and found that it can abrogate the mitotic checkpoint in a dominant negative manner. Last, hMPS1 was found to associate with the anaphase promoting complex, thus raising the possibility that its checkpoint functions extend beyond the kinetochore.
Figures
Figure 1
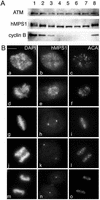
Mitotic phosphorylation and subcellular distribution of hMPS1. (A) hMPS1 is dephosphorylated when cells exit mitosis. HeLa cells that were blocked in mitosis with nocodazole were collected and released into drug-free medium, and samples were taken every 30 min up to 3 h (lanes 1–7; lane 1 is time 0). One sample was released into medium containing the proteosome inhibitor ALLN and harvested 3 h later (lane 8). Equal amounts of protein obtained from the different lysates were probed for hMPS1, cyclin B, and ATM. ATM was used as a loading control because its level has been shown to not fluctuate during the cell cycle (Gately et al., 1998). (B) Affinity-purified rabbit anti-hMPS1 antibodies were used to stain HeLa cells at various stages of mitosis: prophase (a–c), prometaphase (d–f), metaphase (g–i), anaphase (j–l), and telophase (m–o). DAPI and ACA (anticentromere autoimmune serum) were used to stain chromosomes and kinetochores, respectively. Note the bright foci of centrosome staining at spindle poles. (C) hMPS1 is localized to centrosomes and nuclear pores during interphase. Interphase HeLa cells were costained with rabbit anti-hMPS1 and mouse anti-γ tubulin (a–c) to verify hMPS1 at centrosomes. The presence of hMPS1 at nuclear pores was revealed by costaining with mAb414 (d and e). A merged image shows coincident localization of hMPS1 with mAb414 (f). Cells permeabilized with digitonin were stained with hMPS1 and mAb414 antibodies (g–i). Rabbit anti-hMPS1 was visualized with Alexafluor 488 anti-rabbit secondary antibodies. Mouse anti-γ-tubulin and mAb414 were visualized with Texas Red anti-mouse secondary antibodies. DNA was stained with DAPI. Bar, 10 μm.
Figure 1
Mitotic phosphorylation and subcellular distribution of hMPS1. (A) hMPS1 is dephosphorylated when cells exit mitosis. HeLa cells that were blocked in mitosis with nocodazole were collected and released into drug-free medium, and samples were taken every 30 min up to 3 h (lanes 1–7; lane 1 is time 0). One sample was released into medium containing the proteosome inhibitor ALLN and harvested 3 h later (lane 8). Equal amounts of protein obtained from the different lysates were probed for hMPS1, cyclin B, and ATM. ATM was used as a loading control because its level has been shown to not fluctuate during the cell cycle (Gately et al., 1998). (B) Affinity-purified rabbit anti-hMPS1 antibodies were used to stain HeLa cells at various stages of mitosis: prophase (a–c), prometaphase (d–f), metaphase (g–i), anaphase (j–l), and telophase (m–o). DAPI and ACA (anticentromere autoimmune serum) were used to stain chromosomes and kinetochores, respectively. Note the bright foci of centrosome staining at spindle poles. (C) hMPS1 is localized to centrosomes and nuclear pores during interphase. Interphase HeLa cells were costained with rabbit anti-hMPS1 and mouse anti-γ tubulin (a–c) to verify hMPS1 at centrosomes. The presence of hMPS1 at nuclear pores was revealed by costaining with mAb414 (d and e). A merged image shows coincident localization of hMPS1 with mAb414 (f). Cells permeabilized with digitonin were stained with hMPS1 and mAb414 antibodies (g–i). Rabbit anti-hMPS1 was visualized with Alexafluor 488 anti-rabbit secondary antibodies. Mouse anti-γ-tubulin and mAb414 were visualized with Texas Red anti-mouse secondary antibodies. DNA was stained with DAPI. Bar, 10 μm.
Figure 2
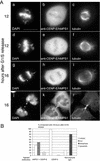
hMPS1 is critical for normal mitotic progression. HeLa cells synchronized at the G1/S boundary were injected with hMPS1 antibodies, fixed 12 and 16 h later, and stained with Cy5 anti-rabbit antibodies to identify the injected cells. Samples at the 12-h time point were also stained with rat anti-MAD1 (a–c) and rat anti-CENP-E (d–f) and counterstained with Alexafluor 488 anti-rat secondary antibodies. At the 16-h time point (g–i), injected cells had divided, as seen by phase-contrast microscopy. DNA was stained with DAPI. The inset shows chromatin bridges or “cut” phenotype in an anaphase cell that had been injected with hMPS1 antibodies. Bar, 10 μm.
Figure 3
Mitotic arrest induced by loss of CENP-E depends on hMPS1. Synchronized HeLa cells were coinjected with CENP-E and hMPS1 antibodies, CENP-E and nonimmune IgG, or nonimmune IgG alone and sampled 12 and 16 h after release from the G1/S boundary. (A) Cells coinjected with CENP-E and hMPS1 antibodies were stained for the injected antibodies and α-tubulin to visualize the spindle and DAPI to visualize chromosomes. At the 12-h time point, the presence of mono-oriented chromosomes indicated a CENP-E defect. At the 16-h time point, the doubly injected cells entered anaphase with chromatin bridges (“cut” phenotype, g) or divided to form multilobed nuclei or micronuclei (j). Bar, 10 μm. (B) Cells injected with different antibodies were fixed at the 16-h time point, visualized by phase-contrast microscopy, and stained with DAPI to determine their fates. Cells in mitosis, those that prematurely exited mitosis with chromatin bridges (“cut” phenotype), those that exited mitosis normally, and apoptotic cells were quantified and compared. An average of 200 cells were counted for each experiment.
Figure 4
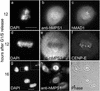
Depletion of hMPS1 by siRNA prevented binding of MAD1 but not BUB1, BUBR1, and hROD to kinetochores. HeLa cells were transfected with hMPS1 siRNA and then processed for immunofluorescence 38 h later. Rat anti-MAD1, BUBR1, BUB1, and hROD antibodies were used with Alexafluor 488–conjugated secondary antibodies. Endogenous hMPS1 was stained with rabbit anti-hMPS1 antibody and Alexafluor 594–conjugated secondary antibody. ACA- and Cy5-labeled anti-human secondary antibody was used to visualize kinetochores, and DAPI was stained for chromosomes. Arrows indicate pairs of centromeres/kinetochores for comparison. Bar, 10 μm.
Figure 5
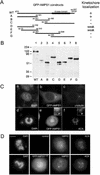
Mapping the kinetochore targeting domain of hMPS1. (A) Schematic depiction of hMPS1 fragments that were used to map the kinetochore binding domain. WT, wild-type. (B) Lysates prepared from HeLa cells transfected with various GFP-hMPS1 constructs were probed with anti-GFP antibodies. (C) Cells transfected with full-length hMPS1 fused to GFP were stained with γ-tubulin (c) or ACA (f). (D) GFP-hMPS11–301 depletes endogenous hMPS1 from kinetochores. An untransfected control cell (top) and a transfected cell (bottom) on the same coverslip were stained with hMPS1 antibody and visualized with Alexafluor 594 conjugated anti-rabbit secondary antibody. DAPI and ACA followed by Cy5 conjugated anti-human secondary antibody were used to stain chromosomes and kinetochores. Bar, 10 μm.
Figure 6
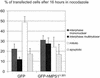
GFP-hMPS11–301 disrupts mitotic checkpoint. HeLa cells were transfected with pECEGFP or pECEGFP-hMPS11–301 at the time of release from double thymidine block, and nocodazole was added 8 h later. After 16 h in nocodazole, transfected cells were examined live by phase-contrast and fluorescence microscopy to determine mitotic index. The average of three experiments is presented here.
Figure 7
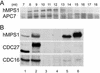
hMPS1 associates with APC in interphase and mitosis. (A) Elution profiles of hMPS1 and APC7 from a Superose 6 column. Asynchronous HeLa cell lysate, ∼1 mg, was loaded onto the column, and 1-ml fractions were collected. The fractions across the elution profile were probed with hMPS1 and APC7 antibodies. (B) hMPS1 coimmunoprecipitated with the APC subunits CDC27 and CDC16 in interphase and mitotic cell lysates. Asynchronous (lanes 1, 3, and 5) or mitotic (lanes 2, 4, and 6) lysates, ∼250 μg, were immunoprecipitated with 1.5 μg nonimmune IgG (lanes 3–4) or anti-hMPS1 antibody (lanes 5–6). Then ∼16 μg of the lysates (1/15 of the amount used for immunoprecipitation) were run in lanes 1 and 2 as controls.
Similar articles
- BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1.
Chen RH. Chen RH. J Cell Biol. 2002 Aug 5;158(3):487-96. doi: 10.1083/jcb.200204048. Epub 2002 Aug 5. J Cell Biol. 2002. PMID: 12163471 Free PMC article. - Phosphorylation at threonine 288 by cell cycle checkpoint kinase 2 (CHK2) controls human monopolar spindle 1 (Mps1) kinetochore localization.
Yeh CW, Yu ZC, Chen PH, Cheng YC, Shieh SY. Yeh CW, et al. J Biol Chem. 2014 May 30;289(22):15319-27. doi: 10.1074/jbc.M114.552273. Epub 2014 Apr 24. J Biol Chem. 2014. PMID: 24764296 Free PMC article. - Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint.
Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbé JC. Abrieu A, et al. Cell. 2001 Jul 13;106(1):83-93. doi: 10.1016/s0092-8674(01)00410-x. Cell. 2001. PMID: 11461704 - Orchestration of the spindle assembly checkpoint by CDK1-cyclin B1.
Hayward D, Alfonso-Pérez T, Gruneberg U. Hayward D, et al. FEBS Lett. 2019 Oct;593(20):2889-2907. doi: 10.1002/1873-3468.13591. Epub 2019 Sep 13. FEBS Lett. 2019. PMID: 31469407 Review. - Leader of the SAC: molecular mechanisms of Mps1/TTK regulation in mitosis.
Pachis ST, Kops GJPL. Pachis ST, et al. Open Biol. 2018 Aug;8(8):180109. doi: 10.1098/rsob.180109. Open Biol. 2018. PMID: 30111590 Free PMC article. Review.
Cited by
- Spindle checkpoint-independent inhibition of mitotic chromosome segregation by Drosophila Mps1.
Althoff F, Karess RE, Lehner CF. Althoff F, et al. Mol Biol Cell. 2012 Jun;23(12):2275-91. doi: 10.1091/mbc.E12-02-0117. Epub 2012 May 2. Mol Biol Cell. 2012. PMID: 22553353 Free PMC article. - VDAC3 regulates centriole assembly by targeting Mps1 to centrosomes.
Majumder S, Slabodnick M, Pike A, Marquardt J, Fisk HA. Majumder S, et al. Cell Cycle. 2012 Oct 1;11(19):3666-78. doi: 10.4161/cc.21927. Epub 2012 Aug 30. Cell Cycle. 2012. PMID: 22935710 Free PMC article. - Autophosphorylation is sufficient to release Mps1 kinase from native kinetochores.
Koch LB, Opoku KN, Deng Y, Barber A, Littleton AJ, London N, Biggins S, Asbury CL. Koch LB, et al. Proc Natl Acad Sci U S A. 2019 Aug 27;116(35):17355-17360. doi: 10.1073/pnas.1901653116. Epub 2019 Aug 12. Proc Natl Acad Sci U S A. 2019. PMID: 31405987 Free PMC article. - Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression.
Fisk HA, Mattison CP, Winey M. Fisk HA, et al. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):14875-80. doi: 10.1073/pnas.2434156100. Epub 2003 Dec 1. Proc Natl Acad Sci U S A. 2003. PMID: 14657364 Free PMC article. - Centriole assembly and the role of Mps1: defensible or dispensable?
Pike AN, Fisk HA. Pike AN, et al. Cell Div. 2011 Apr 14;6:9. doi: 10.1186/1747-1028-6-9. Cell Div. 2011. PMID: 21492451 Free PMC article.
References
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbe JC. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. - PubMed
- Amon A. The spindle checkpoint. Curr Opin Genet Dev. 1999;9:69–75. - PubMed
- Bardin AJ, Visintin R, Amon A. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 2000;102:21–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources