Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells - PubMed (original) (raw)
Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells
Subhajit Dasgupta et al. J Biol Chem. 2003.
Abstract
The presence of neuroantigen-primed T cells recognizing self-myelin antigens within the CNS is necessary for the development of demyelinating autoimmune disease like multiple sclerosis. This study was undertaken to investigate the role of myelin basic protein (MBP)-primed T cells in the expression of proinflammatory cytokines in microglial cells. MBP-primed T cells alone induced specifically the microglial expression of interleukin (IL)-1beta, IL-1alpha tumor necrosis factor alpha, and IL-6, proinflammatory cytokines that are primarily involved in the pathogenesis of MS. This induction was primarily dependent on the contact between MBP-primed T cells and microglia. The activation of microglial NF-kappaB and CCAAT/enhancer-binding protein beta (C/EBPbeta) by MBP-primed T cell contact and inhibition of contact-mediated microglial expression of proinflammatory cytokines by dominant-negative mutants of p65 and C/EBPbeta suggest that MBP-primed T cells induce microglial expression of cytokines through the activation of NF-kappaB and C/EBPbeta. In addition, we show that MBP-primed T cells express very late antigen-4 (VLA-4), and functional blocking antibodies to alpha4 chain of VLA-4 (CD49d) inhibited the ability of MBP-primed T cells to induce microglial proinflammatory cytokines. Interestingly, the blocking of VLA-4 impaired the ability of MBP-primed T cells to induce microglial activation of only C/EBPbeta but not that of NF-kappaB. This study illustrates a novel role of VLA-4 in regulating neuroantigen-primed T cell-induced activation of microglia through C/EBPbeta
Figures
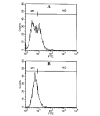
FIG. 1. FACS analysis of BV-2 microglia and MBP-primed T cell coculture with FITC-labeled anti-CD3
Purified MBP-primed T cells were added to BV-2 glial cells (0.5:1 of T cell:glia) under serum-free condition. A, after 1 h of incubation, cells (adherent and non-adherent) were taken out for FACS analysis. B, after 1 h of incubation, culture dishes were shaken and washed three times with HBSS to remove non-adherent cells. Adherent cells were taken out for FACS analysis. Cells were treated with 0.5 ml of appropriately diluted FITC-labeled anti-CD3 for 30 min followed by FACS analysis in a FACScan as described under “Materials and Methods.”
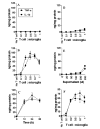
FIG. 2. MBP-primed T cells induce the production of IL-1_β_ and TNF-α in mouse BV-2 microglial cells and primary microglia
BV-2 cells received different concentrations of normal (A) and MBP-primed T cells (B) in direct contact under serum-free condition. After 1 h of incubation, culture dishes were shaken and washed three times with HBSS to remove the burden of T cells. Adherent BV-2 cells then were incubated in serum-free medium for 23 h, and supernatants were used to assay IL-1_β_ and TNF-α as mentioned under “Materials and Methods.” Data are mean ± S.D. of three different experiments. C, BV-2 cells were stimulated with MBP-primed T cells (0.5:1 of T cell:glia) under serum-free condition. After 1 h of incubation, culture dishes were shaken and washed three times with HBSS to remove the burden of T cells. At different hours of incubation in serum-free medium, supernatants were used to assay IL-1_β_ and TNF-α. Data are mean ± S.D. of three different experiments. D, BV-2 cells received different concentrations of MBP-primed T cells within insert under serum-free condition. After 24 h, supernatants were used to assay IL-1_β_ and TNF-α. Data are mean ± S.D. of three different experiments. E, BV-2 cells received different concentrations of conditioned supernatant of MBP-primed T cells under serum-free condition. After 24 h, supernatants were used to assay IL-1_β_ and TNF-α. Data are mean ± S.D. of three different experiments. F, mouse primary microglia received different concentrations of MBP-primed T cells in direct contact under serum-free condition. After 1 h of incubation, culture dishes were shaken and washed three times with HBSS to remove the burden of T cells. Adherent cells were incubated with serum-free medium for another 23 h, and supernatants were used to assay IL-1_β_ and TNF-α. Data are mean ± S.D. of three different experiments.
FIG. 3. MBP-primed T cells induce the expression of different proinflammatory cytokines in mouse BV-2 microglial cells
BV-2 cells were stimulated with either normal (A) or MBP-primed T cells (B) (0.5:1 of T cell:glia) under serum-free condition. After 1 h of stimulation, culture dishes were shaken and washed three times with HBSS to lower T cell concentration. BV-2 cells then received serum-free medium, and after 3 h of incubation, total RNA was isolated from adherent BV-2 cells for the analysis of different proinflammatory and anti-inflammatory cytokines by a gene array kit (GEArray) following manufacturer’s protocol as described under “Materials and Methods.” C, the relative expression of different cytokines (cytokines/glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) was measured after scanning the bands with a Fluor Chem 8800 Imaging System (Alpha Innotech Corporation). Data are expressed as the mean ± S.D. of three different experiments.
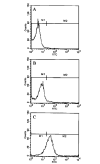
FIG. 4. Expression of VLA-4 on the surface of MBP-primed T cells
Normal or MBP-primed T cells were treated with 0.5 ml of appropriately diluted FITC-labeled anti-VLA _α_4 for 30 min followed by FACS analysis. A, FITC-nonconjugated normal T cells. B, FITC-conjugated normal T cells. C, FITC-conjugated MBP-primed T cells.
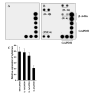
FIG. 5. Functional blocking antibodies against the _α_4 chain of VLA-4 inhibit the ability of MBP-primed T cells to induce the expression of proinflammatory cytokines in BV-2 microglial cells
MBP-primed T cells were mixed with either different concentrations of antibodies against the α_4 chain of VLA-4 or control IgG and rocked gently for 1 h at room temperature. Cells were centrifuged, washed twice, and added to BV-2 microglial cells at a ratio of 0.5:1 T cell:microglia. After 1 h of stimulation, culture dishes were shaken and washed to lower T cell concentration. A, adherent cells were incubated with serum-free medium for another 23 h. Supernatants were used to assay IL-1_β and TNF-α. Data are mean ± S.D. of three different experiments. B, adherent cells were incubated with serum-free medium for another 3 h. The expression of different cytokines was analyzed by a gene array kit (GEArray). The relative expression of different cytokines (cytokines/glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) was measured after scanning the bands with a Fluor Chem 8800 Imaging System. Data are mean ± S.D. of three different experiments.
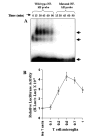
FIG. 6. MBP-primed T cells induce the activation of NF-_κ_B in BV-2 microglial cells
A, cells incubated in serum-free DMEM/F-12 were stimulated with MBP-primed T cells (0.5:1 of T cell:glia). After different minutes of stimulation, culture dishes were shaken and washed three times with HBSS. Adherent cells were taken out to prepare nuclear extracts, and nuclear proteins were used for EMSA as described under “Materials and Methods.” The upper, middle, and lower arrows indicate the induced NF-_κ_B band, nonspecific band, and the unbound probe, respectively. B, cells plated at 50–60% confluence in 12-well plates were cotransfected with 0.5 _μ_g of PBIIX-Luc and 25 ng of pRL-TK using LipofectAMINE Plus as described under “Materials and Methods.” After 24 h of transfection, BV-2 cells were stimulated with different concentrations of MBP-primed T cells. After 1 h of stimulation, T cells were removed followed by the incubation of adherent BV-2 cells in serum-free medium for another 5 h. Firefly and Renilla luciferase activities were obtained by analyzing total cell extract of BV-2 cells as described under “Materials and Methods.” Data are mean ± S.D. of three different experiments.
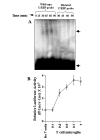
FIG. 7. MBP-primed T cells induce the activation of C/EBP_β_ in BV-2 microglial cells
A, cells incubated in serum-free DMEM/F-12 were stimulated with MBP-primed T cells (0.5:1 of T cell:glia). After different minutes of stimulation, culture dishes were shaken and washed three times with HBSS and EMSA was carried out as described earlier. The upper and lower arrows indicate the induced C/EBP_β_ band and the unbound probe, respectively. B, BV-2 cells plated at 50–60% confluence in 12-well plates were cotransfected with 0.5 μ_g of pC/EBP_β -Luc (an C/EBP_β_ -dependent reporter construct) and 25 ng of pRL-TK. After 24 h of transfection, BV-2 cells were stimulated with different concentrations of MBP-primed T cells. After 1 h of stimulation, T cells were removed followed by the incubation of adherent BV-2 cells in serum-free medium for another 5 h. Firefly and Renilla luciferase activities were obtained by analyzing total cell extract of BV-2 cells. Data are mean ± S.D. of three different experiments.
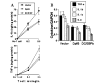
FIG. 8. Dominant-negative mutants of p65 (Δp65) and C/EBP_β_ (ΔC/EBP_β_) inhibit MBP-primed T cell-induced expression of proinflammatory cytokines in BV-2 microglial cells
Cells were transfected with 0.5 μ_g of either Δp65 or ΔC/EBP_β using LipofectAMINE-plus. After 24 h of transfection, cells were stimulated with MBP-primed T cells (0.5:1 of T cell:glia) under serum-free condition. A, after 1 h of stimulation, T cells were removed followed by the incubation of adherent BV-2 cells in serum-free medium for another 23 h. Supernatants were used to assay IL-1_β_ and TNF-α. Data are mean ± S.D. of three different experiments. B, after 1 h of stimulation, T cells were removed followed by the incubation of adherent BV-2 cells in serum-free media for another 3 h. The expression of different cytokines was analyzed by a gene array kit (GEArray). The relative expression of different cytokines (cytokines/glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) was measured after scanning the bands with a Fluor Chem 8800 Imaging System. Data are mean ± S.D. of three different experiments.
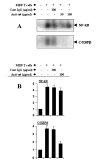
FIG. 9. Functional blocking antibodies against the α_4 chain of VLA-4 inhibit the ability of MBP-primed T cells to induce the activation of C/EBP_β but not that of NF-_κ_B in BV-2 microglial cells
A, MBP-primed T cells were mixed with either different concentrations of antibodies against the _α_4 chain of VLA-4 or control IgG and rocked gently for 1 h at room temperature. Cells were centrifuged, washed three times, and added to BV-2 microglial cells at a ratio of 0.5:1 T cell:microglia. After 1 h of incubation, culture dishes were shaken and washed three times with HBSS and EMSA was carried out to study the DNA binding activity of NF-κ_B (upper panel) and C/EBP_β (lower panel) in adherent microglia. B, BV-2 microglial cells plated at 50–60% confluence in 12-well plates were cotransfected with 0.5 μ_g of either PBIIX-Luc (upper panel) or p C/EBP_β -Luc (lower panel) and 25 ng of pRL-TK. After 24 h of transfection, BV-2 cells were stimulated with either anti-_α_4-preincubated or control IgG-preincubated MBP-primed T cells (0.5:1 of T cell:glia). After 1 h of stimulation, T cells were removed by shaking and washing followed by the incubation of adherent BV-2 cells in serum-free media for another 5 h. Firefly and Renilla luciferase activities were obtained by analyzing total cell extract of BV-2 cells. Data are mean ± S.D. of three different experiments.
Similar articles
- Myelin basic protein-primed T cells of female but not male mice induce nitric-oxide synthase and proinflammatory cytokines in microglia: implications for gender bias in multiple sclerosis.
Dasgupta S, Jana M, Liu X, Pahan K. Dasgupta S, et al. J Biol Chem. 2005 Sep 23;280(38):32609-17. doi: 10.1074/jbc.M500299200. Epub 2005 Jul 26. J Biol Chem. 2005. PMID: 16046404 Free PMC article. - Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells. Implications for multiple sclerosis.
Dasgupta S, Jana M, Liu X, Pahan K. Dasgupta S, et al. J Biol Chem. 2002 Oct 18;277(42):39327-33. doi: 10.1074/jbc.M111841200. Epub 2002 Aug 9. J Biol Chem. 2002. PMID: 12176974 Free PMC article. - Gender-specific expression of beta1 integrin of VLA-4 in myelin basic protein-primed T cells: implications for gender bias in multiple sclerosis.
Brahmachari S, Pahan K. Brahmachari S, et al. J Immunol. 2010 Jun 1;184(11):6103-13. doi: 10.4049/jimmunol.0804356. Epub 2010 May 5. J Immunol. 2010. PMID: 20483780 Free PMC article. - VLA-4 as a Central Target for Modulating Neuroinflammatory Disorders.
Jurberg AD, Chaves B, Pinho LG, da Silva JHM, Savino W, Cotta-de-Almeida V. Jurberg AD, et al. Neuroimmunomodulation. 2021;28(4):213-221. doi: 10.1159/000518721. Epub 2021 Sep 2. Neuroimmunomodulation. 2021. PMID: 34515173 Review. - Microglial diversity by responses and responders.
Gertig U, Hanisch UK. Gertig U, et al. Front Cell Neurosci. 2014 Apr 1;8:101. doi: 10.3389/fncel.2014.00101. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24744702 Free PMC article. Review.
Cited by
- C/EBPβ: A transcription factor associated with the irreversible progression of Alzheimer's disease.
Yao Q, Long C, Yi P, Zhang G, Wan W, Rao X, Ying J, Liang W, Hua F. Yao Q, et al. CNS Neurosci Ther. 2024 Apr;30(4):e14721. doi: 10.1111/cns.14721. CNS Neurosci Ther. 2024. PMID: 38644578 Free PMC article. Review. - Muscle-building supplement β-hydroxy β-methylbutyrate binds to PPARα to improve hippocampal functions in mice.
Paidi RK, Raha S, Roy A, Pahan K. Paidi RK, et al. Cell Rep. 2023 Jul 25;42(7):112717. doi: 10.1016/j.celrep.2023.112717. Epub 2023 Jul 11. Cell Rep. 2023. PMID: 37437568 Free PMC article. - Suppression of Experimental Autoimmune Encephalomyelitis in Mice by β-Hydroxy β-Methylbutyrate, a Body-Building Supplement in Humans.
Sheinin M, Mondal S, Roy A, Gorai S, Rangasamy SB, Poddar J, Pahan K. Sheinin M, et al. J Immunol. 2023 Jul 15;211(2):187-198. doi: 10.4049/jimmunol.2200267. J Immunol. 2023. PMID: 37314416 Free PMC article. - Monocyte biomarkers define sargramostim treatment outcomes for Parkinson's disease.
Abdelmoaty MM, Machhi J, Yeapuri P, Shahjin F, Kumar V, Olson KE, Mosley RL, Gendelman HE. Abdelmoaty MM, et al. Clin Transl Med. 2022 Jul;12(7):e958. doi: 10.1002/ctm2.958. Clin Transl Med. 2022. PMID: 35802825 Free PMC article. - Defining the Innate Immune Responses for SARS-CoV-2-Human Macrophage Interactions.
Abdelmoaty MM, Yeapuri P, Machhi J, Olson KE, Shahjin F, Kumar V, Zhou Y, Liang J, Pandey K, Acharya A, Byrareddy SN, Mosley RL, Gendelman HE. Abdelmoaty MM, et al. Front Immunol. 2021 Oct 4;12:741502. doi: 10.3389/fimmu.2021.741502. eCollection 2021. Front Immunol. 2021. PMID: 34671355 Free PMC article.
References
- Martin R, McFarland HF, McFarlin DE. Annu Rev Immunol. 1992;10:153–187. - PubMed
- Liu H, MacKenzie-Graham AJ, Palaszynski K, Liva S, Voskuhl RR. J Neuroimmunol. 2001;116:83–93. - PubMed
- Iglesias A, Bauer J, Litzenburger T, Schubart A, Linington C. Glia. 2001;36:220–234. - PubMed
- Utz U, McFarland HF. J Neuropathol Exp Neurol. 1994;53:351–358. - PubMed
- Chabot S, Yong VW. Neurology. 2000;55:1497–1505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS039940/NS/NINDS NIH HHS/United States
- R01 NS039940-02/NS/NINDS NIH HHS/United States
- R03 AG019487-01/AG/NIA NIH HHS/United States
- NS39940/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous