Virus-like interference in the latency and prevention of Creutzfeldt-Jakob disease - PubMed (original) (raw)
Virus-like interference in the latency and prevention of Creutzfeldt-Jakob disease
Laura Manuelidis et al. Proc Natl Acad Sci U S A. 2003.
Abstract
We previously showed that intracerebral (ic) inoculation of the attenuated SY strain of Creutzfeld-Jakob disease in mice could delay clinical signs and widespread neuropathology evoked by subsequent ic challenge with the more virulent FU strain. Using lower doses of SY and FU ic, we here demonstrate that mice can be protected well into old age without demonstrable neuropathology or pathologic prion protein (PrP-res). In contrast, parallel FU only controls became terminally diseased 1 year earlier. To determine whether factors elaborated in response to SY might be part of this effect, we evaluated brain and serum samples from additional parallel mice at 90 days after SY infection and just before FU challenge. The infectivity of FU preparations was significantly reduced by mixing with these fresh SY brain homogenates but not by mixing with SY serum samples, suggesting that brain cells were elaborating labile inhibitory factors that were part of the protective response. SY infectivity was too low to be detected in these brain homogenates. Although suppression could be overcome by higher FU doses ic, strong protection against maximal doses of FU was observed by using i.v. inoculations. Because myeloid microglia are infectious and also elaborate many factors in response to the foreign Creutzfeld-Jakob disease agent, it is likely that innate immunity underlies the profound protection shown here. In principle, it should be possible to artificially stimulate relevant myeloid pathways to better prevent and/or delay the clinical and pathological sequelae of these infections.
Figures
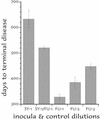
Figure 1
Interference ic with low-dose SY and FU (see text). Incubation of SY-only mice (SY), SY mice challenged with FU at 92 days (SY/FU), normal brain inoculated mice challenged with FU at 92 days (Nl/FU), and mice inoculated only with normal brain on day 0 (Nl). Each inoculated mouse is shown with found dead (fd) animals indicated by arrows. Old surviving mice were killed at 659 days. All of the Nl/FU mice showed clear clinical signs (cli+, dotted curve fit) that closely followed terminal signs (Nl/FU end), whereas SY-only and SY/FU-parallel mice survived >300 days longer, and many of these showed no clinical signs or pathology.
Figure 2
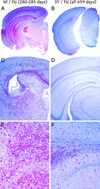
(A, C, and E) Neuropathology of representative mice inoculated ic with normal brain followed by FU challenge at 92 days (Nl/FU), terminal at 280–285 days, are shown, with those first inoculated with SY (SY/FU all killed at 659 days). The low-power images in A and B show widespread pathologic PrP (red) in Nl/FU but not protected SY/FU mice. Widespread activated microglia (keratin sulfate detection, C and D) also reveals widespread microglia only in the unprotected controls. Similarly, obvious vacuolar change amidst many red hypertrophic astrocytes (glial fibrillary acidic protein detection) is seen only in Nl/FU (E) but not in SY/FU (F) mice.
Figure 3
Western blot of representative 10% brain homogenates without PK digestion (left 7 lanes) and the same samples treated with PK (+PK lanes). Each sample was 5 μl. Only the Nl/FU controls at 280 days (FU) show strong PrP-res bands, and the normal (N), SY-only (SY), and SY/FU mice show no detectable PrP-res, including the mouse at 635 days with SY clinical signs. SYp lane shows PrP-res in the early-passage SY brain, and the last SY lane shows no PrP-res in SY brains at 90 days, just before FU challenge.
Figure 4
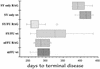
Box plot of incubation times with higher doses of FU and later passaged SY in WT and RAG-1 mice. Median is shown by line in each box, and the circle represents an outlier mouse. Interference is completely abolished except in the SY/FU wt mice above the median (see text).
Figure 5
Interference experiment using the i.v. route. Maximal doses (10% brain homogenates) of low-passage SY and FU (SY-1/FU-1) are 193 days longer (mean ± SEM) than unprotected FU-1 controls. They were even 73 longer than 100-fold dilutions of FU (FU-3), indicating a strong suppression of FU by the i.v. route. The SY-only controls (SY-1) with more surviving mice (at 698 days) were, however, somewhat longer-lived than the SY/FU mice.
Similar articles
- Two Creutzfeldt-Jakob disease agents reproduce prion protein-independent identities in cell cultures.
Arjona A, Simarro L, Islinger F, Nishida N, Manuelidis L. Arjona A, et al. Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8768-73. doi: 10.1073/pnas.0400158101. Epub 2004 May 25. Proc Natl Acad Sci U S A. 2004. PMID: 15161970 Free PMC article. - Vaccination with an attenuated Creutzfeldt-Jakob disease strain prevents expression of a virulent agent.
Manuelidis L. Manuelidis L. Proc Natl Acad Sci U S A. 1998 Mar 3;95(5):2520-5. doi: 10.1073/pnas.95.5.2520. Proc Natl Acad Sci U S A. 1998. PMID: 9482918 Free PMC article. - Attenuated Creutzfeldt-Jakob Disease agents can hide more virulent infections.
Manuelidis L, Yun Lu Z. Manuelidis L, et al. Neurosci Lett. 2000 Nov 3;293(3):163-6. doi: 10.1016/s0304-3940(00)01514-7. Neurosci Lett. 2000. PMID: 11036186 - A 25 nm virion is the likely cause of transmissible spongiform encephalopathies.
Manuelidis L. Manuelidis L. J Cell Biochem. 2007 Mar 1;100(4):897-915. doi: 10.1002/jcb.21090. J Cell Biochem. 2007. PMID: 17044041 Review. - Prion encephalopathies of animals and humans.
Prusiner SB. Prusiner SB. Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
Cited by
- Two Creutzfeldt-Jakob disease agents reproduce prion protein-independent identities in cell cultures.
Arjona A, Simarro L, Islinger F, Nishida N, Manuelidis L. Arjona A, et al. Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8768-73. doi: 10.1073/pnas.0400158101. Epub 2004 May 25. Proc Natl Acad Sci U S A. 2004. PMID: 15161970 Free PMC article. - Prion strain interactions are highly selective.
Nilsson KP, Joshi-Barr S, Winson O, Sigurdson CJ. Nilsson KP, et al. J Neurosci. 2010 Sep 8;30(36):12094-102. doi: 10.1523/JNEUROSCI.2417-10.2010. J Neurosci. 2010. PMID: 20826672 Free PMC article. - Environmental and host factors that contribute to prion strain evolution.
Bartz JC. Bartz JC. Acta Neuropathol. 2021 Jul;142(1):5-16. doi: 10.1007/s00401-021-02310-6. Epub 2021 Apr 25. Acta Neuropathol. 2021. PMID: 33899132 Free PMC article. Review. - Scrapie protein degradation by cysteine proteases in CD11c+ dendritic cells and GT1-1 neuronal cells.
Luhr KM, Nordström EK, Löw P, Ljunggren HG, Taraboulos A, Kristensson K. Luhr KM, et al. J Virol. 2004 May;78(9):4776-82. doi: 10.1128/jvi.78.9.4776-4782.2004. J Virol. 2004. PMID: 15078959 Free PMC article. - Transmissible encephalopathy agents: virulence, geography and clockwork.
Manuelidis L. Manuelidis L. Virulence. 2010 Mar-Apr;1(2):101-4. doi: 10.4161/viru.1.2.10822. Virulence. 2010. PMID: 21178425 Free PMC article.
References
- Oesch B D, Westaway D, Wälchli M, McKinley M P, Kent S B, Aebersold R, Barry R A, Tempst P, Teplow D E, Hood L E, et al. Cell. 1985;40:735–746. - PubMed
- Manuelidis L. Ann NY Acad Sci. 1994;724:259–281. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials