Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice - PubMed (original) (raw)
Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice
Rima Koka et al. J Exp Med. 2003.
Abstract
Natural killer (NK) cells protect hosts against viral pathogens and transformed cells. IL-15 is thought to play a critical role in NK cell development, but its role in the regulation of peripheral NK cells is less well defined. We now find that adoptive transfer of normal NK cells into mice lacking the high affinity interleukin (IL)-15 receptor, IL-15Ralpha, surprisingly results in the abrupt loss of these cells. Moreover, IL-15Ralpha-deficient NK cells can differentiate successfully in radiation bone marrow chimera bearing normal cells. Finally, adoptively transferred IL-15Ralpha-deficient NK cells survive in normal but not IL-15Ralpha-deficient mice. These findings demonstrate that NK cell-independent IL-15Ralpha expression is critical for maintaining peripheral NK cells, while IL-15Ralpha expression on NK cells is not required for this function.
Figures
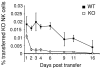
Figure 1.
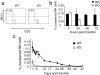
Adoptively transferred NK cells survive significantly longer in a WT host than in a KO host. (a) Representative FACS® plot of transferred WT NK cells in WT (left) and KO (right) hosts. Splenic WT NK cells were transferred intravenously into either WT or KO hosts, congenic for the Ly5 marker. 2 d after transfer, peripheral blood from these mice were analyzed for presence of NK cells using antibodies against Ly5.1, Ly5.2, NK1.1, and CD3. Events displayed are gated on donor cells among the lymphoid gate and presented as the percentage of NK cells among donor cells for this and all subsequent figures. (b) Peripheral blood (PBL) analysis of adoptively transferred WT NK cells in WT (▪) or KO (□) mice at indicated times after transfer. Data presented is the percentage of transferred NK cells/total lymphoid cells collected for this and all subsequent NK cell transfer experiments. (c) Long term PBL analysis of adoptively transferred WT NK cells in WT (▪) or KO (□) mice. Data are representative of three experiments, with three mice in each experiments.
Figure 2.
The disappearance of adoptively transferred NK cells from peripheral blood is not due to differential homing to specific tissues. Quantitation of adoptively transferred WT NK cells from various tissues, (a) spleen, (b) LN, (c) PBL, (d) peritoneum, (e) liver, (f) lung of WT (left) or KO (right) mice 48 h after adoptive transfer. Events displayed in histogram represent percentage of transferred CD3− NK1.1+ cells within the lymphoid gate. Data are representative of two experiments with two mice each.
Figure 3.
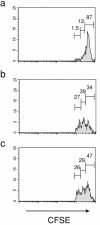
Adoptively transferred splenic NK cells do not proliferate in the absence of specific stimuli. (a) Proliferation of WT NK cells in response to PBS 4 d after transfer into WT host. Events displayed in histogram represent transferred CD3− NK1.1+ cells obtained from the peritoneum of recipient mice. (b) Proliferation of WT NK cells in response to IL-15 4 d after transfer into WT host. (c) Proliferation of WT NK cells in response to IL-15 (5 μg/day) 4 d after transfer into KO host.
Figure 4.
WT and KO mice express similar amounts of IL-15 mRNA. Reverse transcriptase-PCR analysis of secreted and nonsecreted forms of IL-15 mRNA was performed on splenic RNA from mice treated with poly I:C for the indicated number of days.
Figure 5.
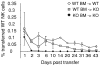
Long term survival of transferred WT NK cells in WT BM→KO radiation BM chimera ⋄ WT BM→WT, ▪ WT BM→KO, ♦ KO BM→KO. Radiation BM chimeric mice were prepared as described in Materials and Methods, allowed to recover for at least 8 wk, and were then used as recipients for WT splenic NK cells. Adoptively transferred cells were congenic for the radiation sensitive compartment of the chimeric mice. Data are representative of two experiments, with two mice each.
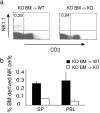
Figure 6.
KO NK cells can survive in the peripheral tissues of KO BM→WT chimera mice. Radiation BM chimeric mice were prepared as described in Materials and Methods and allowed to recover for at least 8 wk before analysis. (a) Representative FACS® plot of BM-derived NK cells in spleens of KO BM→WT (top panel) versus KO BM→KO chimera (bottom panel). (b) Percentage of BM derived NK cells obtained from spleens and peripheral blood of KO BM→WT (▪) and KO BM→KO (□) chimera. Data are representative of five mice.
Figure 7.
IL-15Rα on NK cells is not required for their peripheral maintenance. Long term serial peripheral blood analysis of adoptively transferred KO NK cells after transfer into either WT (▪) or KO (□) hosts. Data are representative of two experiments, with two mice each.
Similar articles
- Differential roles for IL-15R alpha-chain in NK cell development and Ly-49 induction.
Kawamura T, Koka R, Ma A, Kumar V. Kawamura T, et al. J Immunol. 2003 Nov 15;171(10):5085-90. doi: 10.4049/jimmunol.171.10.5085. J Immunol. 2003. PMID: 14607906 - Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis.
Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Burkett PR, et al. J Exp Med. 2004 Oct 4;200(7):825-34. doi: 10.1084/jem.20041389. Epub 2004 Sep 27. J Exp Med. 2004. PMID: 15452177 Free PMC article. - In vivo survival and homeostatic proliferation of natural killer cells.
Prlic M, Blazar BR, Farrar MA, Jameson SC. Prlic M, et al. J Exp Med. 2003 Apr 21;197(8):967-76. doi: 10.1084/jem.20021847. Epub 2003 Apr 14. J Exp Med. 2003. PMID: 12695488 Free PMC article. - The roles of interleukin-15 receptor alpha: trans-presentation, receptor component, or both?
Schluns KS, Stoklasek T, Lefrançois L. Schluns KS, et al. Int J Biochem Cell Biol. 2005 Aug;37(8):1567-71. doi: 10.1016/j.biocel.2005.02.017. Epub 2005 Mar 5. Int J Biochem Cell Biol. 2005. PMID: 15896666 Review. - Increasing the biological activity of IL-2 and IL-15 through complexing with anti-IL-2 mAbs and IL-15Rα-Fc chimera.
Votavova P, Tomala J, Kovar M. Votavova P, et al. Immunol Lett. 2014 May-Jun;159(1-2):1-10. doi: 10.1016/j.imlet.2014.01.017. Epub 2014 Feb 7. Immunol Lett. 2014. PMID: 24512738 Review.
Cited by
- Transcription Factors Associated With IL-15 Cytokine Signaling During NK Cell Development.
Wang X, Zhao XY. Wang X, et al. Front Immunol. 2021 Mar 18;12:610789. doi: 10.3389/fimmu.2021.610789. eCollection 2021. Front Immunol. 2021. PMID: 33815365 Free PMC article. Review. - The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling.
Spits H, Di Santo JP. Spits H, et al. Nat Immunol. 2011 Jan;12(1):21-7. doi: 10.1038/ni.1962. Epub 2010 Nov 28. Nat Immunol. 2011. PMID: 21113163 Review. - IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation.
Mortier E, Woo T, Advincula R, Gozalo S, Ma A. Mortier E, et al. J Exp Med. 2008 May 12;205(5):1213-25. doi: 10.1084/jem.20071913. Epub 2008 May 5. J Exp Med. 2008. PMID: 18458113 Free PMC article. - Homeostasis of IL-15 dependent lymphocyte subsets in the liver.
Cepero-Donates Y, Rakotoarivelo V, Mayhue M, Ma A, Chen YG, Ramanathan S. Cepero-Donates Y, et al. Cytokine. 2016 Jun;82:95-101. doi: 10.1016/j.cyto.2015.12.012. Epub 2016 Jan 5. Cytokine. 2016. PMID: 26778709 Free PMC article. - CD56bright NK IL-7Rα expression negatively associates with HCV level, and IL-7-induced NK function is impaired during HCV and HIV infections.
Judge CJ, Kostadinova L, Sherman KE, Butt AA, Falck-Ytter Y, Funderburg NT, Landay AL, Lederman MM, Sieg SF, Sandberg JK, Anthony DD. Judge CJ, et al. J Leukoc Biol. 2017 Jul;102(1):171-184. doi: 10.1189/jlb.5A1116-456R. Epub 2017 Apr 11. J Leukoc Biol. 2017. PMID: 28400540 Free PMC article.
References
- Karlhofer, F.M., R.K. Ribaudo, and W.M. Yokoyama. 1992. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 358:66–70. - PubMed
- Fehniger, T.A., K. Suzuki, J.B. VanDeusen, M.A. Cooper, A.G. Freud, and M.A. Caligiuri. 2001. Fatal leukemia in interleukin-15 transgenic mice. Blood Cells Mol. Dis. 27:223–230. - PubMed
- Fehniger, T.A., K. Suzuki, A. Ponnappan, J.B. VanDeusen, M.A. Cooper, S.M. Florea, A.G. Freud, M.L. Robinson, J. Durbin, and M.A. Caligiuri. 2001. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J. Exp. Med. 193:219–231. - PMC - PubMed
- Bamford, R.N., A.J. Grant, J.D. Burton, C. Peters, G. Kurys, C.K. Goldman, J. Brennan, E. Roessler, and T.A. Waldmann. 1994. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA. 91:11:4940–4944. - PMC - PubMed
- Burton, J.D., R.N. Bamford, C. Peters, A.J. Grant, G. Kurys, C.K. Goldman, J. Brennan, E. Roessler, and T.A. Waldmann. 1994. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc. Natl. Acad. Sci. USA. 91:4935–4939. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI07090/AI/NIAID NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- AI45860/AI/NIAID NIH HHS/United States
- GM07281/GM/NIGMS NIH HHS/United States
- R01 AI045860/AI/NIAID NIH HHS/United States
- T32 GM007281/GM/NIGMS NIH HHS/United States
- DK42086/DK/NIDDK NIH HHS/United States
- T32 AI007090/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases