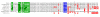TOX defines a conserved subfamily of HMG-box proteins - PubMed (original) (raw)
Comparative Study
TOX defines a conserved subfamily of HMG-box proteins
Emmett O'Flaherty et al. BMC Genomics. 2003.
Abstract
Background: HMG-box proteins are a large and diverse superfamily of architectural factors that share one or more copies of a sequence- and structurally-related DNA binding domain. These proteins can modify chromatin structure by bending and unwinding DNA. HMG-box proteins can be divided into two subfamilies based on whether they recognize DNA in a sequence-dependent or sequence-independent manner. We recently identified an HMG-box protein involved in T cell development, designated TOX, which is highly conserved in humans and mice.
Results: We show here that based on sequence alignment, TOX best fits into the sequence-independent HMG-box family. Three other human and murine predicted proteins are identified that share a common HMG-box domain with TOX, as well as other features. The gene encoding one of these additional family members has a distinct but overlapping pattern of tissue expression when compared to TOX. In addition, we identify genes encoding predicted TOX HMG-box subfamily members in pufferfish and mosquito.
Conclusions: We have identified a novel subfamily of HMG-box proteins that is related to the recently described TOX protein. The highly conserved nature of the TOX family of proteins in humans and mice and differences in the pattern of expression between family members suggest non-overlapping functions of individual proteins. In addition, our data suggest that the TOX subtype of HMG-box domain first appeared in invertebrates, was duplicated in early vertebrates and likely took on new functions in mammalian species.
Figures
Figure 1
Comparison of HMG-box sequences. The HMG-box region of TOX is aligned with the HMG-box motifs found in seven other proteins representing the two major subfamilies of HMG-box proteins. Similarities between the HMG-boxes of both groups of proteins are highlighted in gray and include matches to the consensus HMG-box domain (accession number pf00505) defined in the Pfam protein families' database. Residues in red and purple distinguish these two subgroups of HMG-box proteins and are discussed in the text. In addition, the consensus sequence GXXW (or more rarely AXXW, as also found in TOX) found commonly in HMG-boxes is shown in blue.
Figure 2
Murine TOX subfamily members and their human homologues. (A) The HMG-box and putative NLS regions within each predicted protein are shown in blue and green, respectively. The predicted size and molecular weight of each protein and the chromosomal location of the gene that encodes the protein are also indicated. *Note that the predicted protein LOC241768 as it currently exists in the NCBI database has been modified, and there is some question as to chromosomal location (see Methods). (B) Pair-wise comparison of HMG-box domains of murine and human TOX subfamily members. The upper predicted protein of each pair is mouse derived. Residues that differ from TOX are highlighted in red and the AXXW motif is highlighted in blue. The vertical line represents the position of the conserved exon boundary of the respective genes. (C) Comparison of predicted NLS of murine and human TOX subfamily members. The consensus motif is highlighted in green.
Figure 3
Comparison of TOX and LCP1. (A) Graphical representation of an unfiltered BLAST alignment comparing TOX and LCP1 proteins. Breaks in the proteins to allow maximum alignment are represented by a line. Residues in TOX that are identical to the aligned LCP1 protein are shown in black, while differences are shown in red. The NLS and HMG-box regions are indicated by green and blue bars, respectively. (B) Northern analysis of LCP1 gene expression in normalized poly-A+ RNA isolated from various tissues. The signal obtained from a probe to the housekeeping GAPDH gene is also shown as a loading control.
Figure 4
Identification of TOX subfamily predicted proteins in pufferfish and mosquito. Shown is an amino acid comparison of the TOX NLS and HMG-box regions with predicted proteins encoded in the Fugu rubripes or Anopheles gambiae genomes. Fugu rubripes predicted proteins are designated here simply by the scaffold (Scaf) location of the corresponding gene (see Methods). Amino acids that differ from the TOX HMG-box are highlighted in red, the putative NLS regions are highlighted in green, and the AXXW motif is highlighted in blue. The vertical line represents the position of the conserved exon boundary of the respective genes. Alternative amino acids found in other mammalian family members are also shown (family).
Similar articles
- The Arabidopsis genome encodes structurally and functionally diverse HMGB-type proteins.
Grasser M, Lentz A, Lichota J, Merkle T, Grasser KD. Grasser M, et al. J Mol Biol. 2006 May 5;358(3):654-64. doi: 10.1016/j.jmb.2006.02.068. Epub 2006 Mar 10. J Mol Biol. 2006. PMID: 16563436 - A new mode of DNA binding distinguishes Capicua from other HMG-box factors and explains its mutation patterns in cancer.
Forés M, Simón-Carrasco L, Ajuria L, Samper N, González-Crespo S, Drosten M, Barbacid M, Jiménez G. Forés M, et al. PLoS Genet. 2017 Mar 9;13(3):e1006622. doi: 10.1371/journal.pgen.1006622. eCollection 2017 Mar. PLoS Genet. 2017. PMID: 28278156 Free PMC article. - TOX: an HMG box protein implicated in the regulation of thymocyte selection.
Wilkinson B, Chen JY, Han P, Rufner KM, Goularte OD, Kaye J. Wilkinson B, et al. Nat Immunol. 2002 Mar;3(3):272-80. doi: 10.1038/ni767. Epub 2002 Feb 19. Nat Immunol. 2002. PMID: 11850626 - Role, function and regulation of the thymocyte selection-associated high mobility group box protein in CD8+ T cell exhaustion.
Cheng Y, Shao Z, Chen L, Zheng Q, Zhang Q, Ding W, Zhang M, Yu Q, Gao D. Cheng Y, et al. Immunol Lett. 2021 Jan;229:1-7. doi: 10.1016/j.imlet.2020.11.004. Epub 2020 Nov 11. Immunol Lett. 2021. PMID: 33186634 Review. - The HMG-box: a versatile protein domain occurring in a wide variety of DNA-binding proteins.
Stros M, Launholt D, Grasser KD. Stros M, et al. Cell Mol Life Sci. 2007 Oct;64(19-20):2590-606. doi: 10.1007/s00018-007-7162-3. Cell Mol Life Sci. 2007. PMID: 17599239 Free PMC article. Review.
Cited by
- TCF-1 and TOX regulate the memory formation of intestinal group 2 innate lymphoid cells in asthma.
Bao K, Gu X, Song Y, Zhou Y, Chen Y, Yu X, Yuan W, Shi L, Zheng J, Hong M. Bao K, et al. Nat Commun. 2024 Sep 8;15(1):7850. doi: 10.1038/s41467-024-52252-2. Nat Commun. 2024. PMID: 39245681 Free PMC article. - TOX2 nuclear-cytosol translocation is linked to leukemogenesis of acute T-cell leukemia by repressing TIM3 transcription.
Li A, Zhang J, Zhan L, Liu X, Zeng X, Zhu Q, Wang Z, Li J. Li A, et al. Cell Death Differ. 2024 Nov;31(11):1506-1518. doi: 10.1038/s41418-024-01352-z. Epub 2024 Jul 30. Cell Death Differ. 2024. PMID: 39080376 Free PMC article. - Transcription factor Tox2 is required for metabolic adaptation and tissue residency of ILC3 in the gut.
Das A, Martinez-Ruiz GU, Bouladoux N, Stacy A, Moraly J, Vega-Sendino M, Zhao Y, Lavaert M, Ding Y, Morales-Sanchez A, Harly C, Seedhom MO, Chari R, Awasthi P, Ikeuchi T, Wang Y, Zhu J, Moutsopoulos NM, Chen W, Yewdell JW, Shapiro VS, Ruiz S, Taylor N, Belkaid Y, Bhandoola A. Das A, et al. Immunity. 2024 May 14;57(5):1019-1036.e9. doi: 10.1016/j.immuni.2024.04.001. Epub 2024 Apr 26. Immunity. 2024. PMID: 38677292 - Microfluidics-enabled fluorinated assembly of EGCG-ligands-siTOX nanoparticles for synergetic tumor cells and exhausted t cells regulation in cancer immunotherapy.
Han X, Zhang G, Wu X, Xu S, Liu J, Wang K, Liu T, Wu P. Han X, et al. J Nanobiotechnology. 2024 Mar 4;22(1):90. doi: 10.1186/s12951-024-02328-4. J Nanobiotechnology. 2024. PMID: 38439048 Free PMC article. - Tox4 regulates transcriptional elongation and reinitiation during murine T cell development.
Wang T, Zhao R, Zhi J, Liu Z, Wu A, Yang Z, Wang W, Ni T, Jing L, Yu M. Wang T, et al. Commun Biol. 2023 Jun 7;6(1):613. doi: 10.1038/s42003-023-04992-y. Commun Biol. 2023. PMID: 37286708 Free PMC article.
References
- Goodwin GH, Johns EW. Isolation and characterisation of two calf-thymus chromatin non-histone proteins with high contents of acidic and basic amino acids. Eur J Biochem. 1973;40:215–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous