Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure - PubMed (original) (raw)
Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure
Eric Durand et al. J Bacteriol. 2003 May.
Abstract
The type II secretion pathway of Pseudomonas aeruginosa is involved in the extracellular release of various toxins and hydrolytic enzymes such as exotoxin A and elastase. This pathway requires the function of a macromolecular complex called the Xcp secreton. The Xcp secreton shares many features with the machinery involved in type IV pilus assembly. More specifically, it involves the function of five pilin-like proteins, the XcpT-X pseudopilins. We show that, upon overexpression, the XcpT pseudopilin can be assembled in a pilus, which we call a type II pseudopilus. Image analysis and filtering of electron micrographs indicated that these appendages are composed of individual fibrils assembled together in a bundle structure. Our observations thus revealed that XcpT has properties similar to those of type IV pilin subunits. Interestingly, the assembly of the type II pseudopilus is not exclusively dependent on the Xcp machinery but can be supported by other similar machineries, such as the Pil (type IV pilus) and Hxc (type II secretion) systems of P. aeruginosa. In addition, heterologous pseudopilins can be assembled by P. aeruginosa into a type II pseudopilus. Finally, we showed that assembly of the type II pseudopilus confers increased bacterial adhesive capabilities. These observations confirmed the ability of pseudopilins to form a pilus structure and raise questions with respect to their function in terms of secretion and adhesion, two crucial biological processes in the course of bacterial infections.
Figures
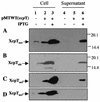
FIG. 1.
XcpT overproduction and exposition at the bacterial cell surface. XcpT was released by the shearing of P. aeruginosa. (A) PAO1 strain bearing pMTWT (P. aeruginosa xcpT); (B) PAO1 strain bearing pMTA3 (P. alcaligenes xcpT); (C) P. aeruginosa xcpRS/hxcR mutant strain bearing pMTWT; (D) P. aeruginosa xcpRS/hxcR/pilQ mutant strain bearing pMTWT. Samples were analyzed by SDS-PAGE and immunoblotting. Lanes 1 to 3, bacterial pellet; lanes 4 to 6, extracellular-appendage-enriched supernatant. The position of XcpT has been indicated, with the use of “aer” or “alc” in subscript to indicate whether the pseudopilin is from P. aeruginosa or P. alcaligenes, respectively.
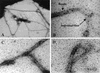
FIG. 2.
XcpT is assembled in multifibrillar structures. The results of negative staining and TEM analysis of a PAO1 strain that overproduces XcpT are shown. See the text for details.
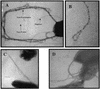
FIG. 3.
XcpT pseudopilin is a major component of the type II pseudopilus. Shown are the results of TEM analysis of PAO1/pMTWT after immunogold labeling with antibodies raised against XcpT. (A, C, and D) The type II pseudopili are seen attached to bacterial cells. (B) A type II pseudopilus with a loop structure is seen enlarged. In panel A, the positions of a type IV pilus, the flagellum, and a type II pseudopilus have been indicated.
FIG. 4.
Image treatment of electron micrographs of a negatively stained portion of the type II pseudopilus. (A) Original image; (B) Fourier transformation of the original image; (C) filtered image; (D) enlargement of the filtered image showing the type II pseudopilus substructure. The length of the observed longitudinal repeat (4.3 nm) and the approximate width of the electron-transparent fibril (3.5 nm) have been indicated.
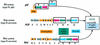
FIG. 5.
Schematic representation of the pil, xcp, and hxc gene clusters. Genes are represented with arrows that reflect their transcription orientation. The genes encoding pilins and pseudopilins are represented in orange. The black boxes represent the regions encoding the conserved N-terminal ends of these proteins. The blue, red, and green genes encode components that are conserved between the three systems. The grey genes are found only in the xcp and hxc systems. The gene encoding the XcpA/PilD protein is unique and is found at the pil locus on the chromosome. However, it is represented apart since it is essential for the function of all three systems.
FIG. 6.
XcpT overproduction interferes with Xcp-dependent secretion. PAO1 strains containing pMTWT (xcpT gene cloned into pMMB190) where indicated or pMMB190 were grown on skim-milk plates in the presence of 2 mM IPTG where indicated. (A and B) A loop of cells was collected from plates and analyzed for XcpT and XcpP production by Western blotting with appropriate antibodies. (C) The zones of hydrolysis observed around the colonies are mostly due to the proteolytic activity of elastase (LasB).
FIG. 7.
Attachment of P. aeruginosa PAO1 and PAK_pilA/fliC_ strains that overproduce (with pMTWT, which contains the xcpT gene) or do not overproduce (with pMMB190, cloning vector) the XcpT pseudopilin. The formation of bacterial film on the walls of Falcon tubes is visualized after crystal violet staining.
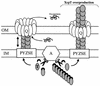
FIG. 8.
Model for Xcp-dependent secretion and assembly of the type II pseudopilus. Under physiological conditions and upon removal of the N-terminal leader peptide (black crescent) by the prepilin peptidase XcpA (hexagon labeled “A”), pseudopilins, such as XcpT (in grey), are assembled into the Xcp secreton. The secreton is also composed of an inner membrane (IM) platform (white boxes labeled “PYZE”) and of the homomultimeric ring containing the outer membrane (OM) secretin XcpQ (Q). Under standard conditions (left part), the structure formed by the pseudopilins is proposed to be transperiplasmic. The positioning of the type II pseudopilus into the secretin channel might be part of a mechanism that prevents leakage from the periplasm through this large pore. The length of the type II pseudopilus might be controlled by the incorporation of minor pseudopilin subunits. The incorporation of the XcpX pseudopilin might stop type II pseudopilus elongation, as was previously proposed (6). Finally, the interaction of the Xcp machinery with exoproteins might somehow induce type II pseudopilus disassembly (two-headed arrow), give access to the channel, and allow the release of exoproteins into the extracellular medium. However, upon overproduction of the XcpT pseudopilin, an abnormally large type II pseudopilus that is exposed to the bacterial cell surface, probably through the XcpQ secretin, is formed (right part).
Similar articles
- XcpX controls biogenesis of the Pseudomonas aeruginosa XcpT-containing pseudopilus.
Durand E, Michel G, Voulhoux R, Kürner J, Bernadac A, Filloux A. Durand E, et al. J Biol Chem. 2005 Sep 9;280(36):31378-89. doi: 10.1074/jbc.M505812200. Epub 2005 Jul 12. J Biol Chem. 2005. PMID: 16012171 - The assembly mode of the pseudopilus: a hallmark to distinguish a novel secretion system subtype.
Durand E, Alphonse S, Brochier-Armanet C, Ball G, Douzi B, Filloux A, Bernard C, Voulhoux R. Durand E, et al. J Biol Chem. 2011 Jul 8;286(27):24407-16. doi: 10.1074/jbc.M111.234278. Epub 2011 May 17. J Biol Chem. 2011. PMID: 21586577 Free PMC article. - Structure of the Pseudomonas aeruginosa XcpT pseudopilin, a major component of the type II secretion system.
Alphonse S, Durand E, Douzi B, Waegele B, Darbon H, Filloux A, Voulhoux R, Bernard C. Alphonse S, et al. J Struct Biol. 2010 Jan;169(1):75-80. doi: 10.1016/j.jsb.2009.09.003. Epub 2009 Sep 9. J Struct Biol. 2010. PMID: 19747550 - Chaperone-assisted assembly and molecular architecture of adhesive pili.
Hultgren SJ, Normark S, Abraham SN. Hultgren SJ, et al. Annu Rev Microbiol. 1991;45:383-415. doi: 10.1146/annurev.mi.45.100191.002123. Annu Rev Microbiol. 1991. PMID: 1683764 Review. - The type II secretion system - a dynamic fiber assembly nanomachine.
Campos M, Cisneros DA, Nivaskumar M, Francetic O. Campos M, et al. Res Microbiol. 2013 Jul-Aug;164(6):545-55. doi: 10.1016/j.resmic.2013.03.013. Epub 2013 Mar 27. Res Microbiol. 2013. PMID: 23542426 Review.
Cited by
- On the path to uncover the bacterial type II secretion system.
Douzi B, Filloux A, Voulhoux R. Douzi B, et al. Philos Trans R Soc Lond B Biol Sci. 2012 Apr 19;367(1592):1059-72. doi: 10.1098/rstb.2011.0204. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22411978 Free PMC article. Review. - The XcpV/GspI pseudopilin has a central role in the assembly of a quaternary complex within the T2SS pseudopilus.
Douzi B, Durand E, Bernard C, Alphonse S, Cambillau C, Filloux A, Tegoni M, Voulhoux R. Douzi B, et al. J Biol Chem. 2009 Dec 11;284(50):34580-9. doi: 10.1074/jbc.M109.042366. Epub 2009 Oct 14. J Biol Chem. 2009. PMID: 19828448 Free PMC article. - XpsE oligomerization triggered by ATP binding, not hydrolysis, leads to its association with XpsL.
Shiue SJ, Kao KM, Leu WM, Chen LY, Chan NL, Hu NT. Shiue SJ, et al. EMBO J. 2006 Apr 5;25(7):1426-35. doi: 10.1038/sj.emboj.7601036. Epub 2006 Mar 9. EMBO J. 2006. PMID: 16525507 Free PMC article. - In vivo cross-linking of EpsG to EpsL suggests a role for EpsL as an ATPase-pseudopilin coupling protein in the Type II secretion system of Vibrio cholerae.
Gray MD, Bagdasarian M, Hol WG, Sandkvist M. Gray MD, et al. Mol Microbiol. 2011 Feb;79(3):786-98. doi: 10.1111/j.1365-2958.2010.07487.x. Mol Microbiol. 2011. PMID: 21255118 Free PMC article. - Calcium is essential for the major pseudopilin in the type 2 secretion system.
Korotkov KV, Gray MD, Kreger A, Turley S, Sandkvist M, Hol WG. Korotkov KV, et al. J Biol Chem. 2009 Sep 18;284(38):25466-70. doi: 10.1074/jbc.C109.037655. Epub 2009 Jul 29. J Biol Chem. 2009. PMID: 19640838 Free PMC article.
References
- Ball, G., E. Durand, A. Lazdunski, and A. Filloux. 2002. A novel type II secretion system in Pseudomonas aeruginosa. Mol. Microbiol. 43:475-485. - PubMed
- Bally, M., A. Filloux, M. Akrim, G. Ball, A. Lazdunski, and J. Tommassen. 1992. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol. Microbiol. 6:1121-1131. - PubMed
- Bitter, W., M. Koster, M. Latijnhouwers, H. de Cock, and J. Tommassen. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27:209-219. - PubMed
- Bitter, W., and J. Tommassen. 1999. Ushers and other doorkeepers. Trends Microbiol. 7:4-6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous