Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration - PubMed (original) (raw)
Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration
Peter Teismann et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Parkinson's disease (PD) is a neurodegenerative disorder of uncertain pathogenesis characterized by the loss of the nigrostriatal dopaminergic neurons, which can be modeled by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Increased expression of cyclooxygenase type 2 (COX-2) and production of prostaglandin E(2) have been implicated in neurodegeneration in several pathological settings. Here we show that COX-2, the rate-limiting enzyme in prostaglandin E(2) synthesis, is up-regulated in brain dopaminergic neurons of both PD and MPTP mice. COX-2 induction occurs through a JNKc-Jun-dependent mechanism after MPTP administration. We demonstrate that targeting COX-2 does not protect against MPTP-induced dopaminergic neurodegeneration by mitigating inflammation. Instead, we provide evidence that COX-2 inhibition prevents the formation of the oxidant species dopamine-quinone, which has been implicated in the pathogenesis of PD. This study supports a critical role for COX-2 in both the pathogenesis and selectivity of the PD neurodegenerative process. Because of the safety record of the COX-2 inhibitors, and their ability to penetrate the blood-brain barrier, these drugs may be therapies for PD.
Figures
Figure 1
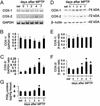
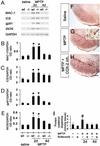
Ventral midbrain COX-1 and COX-2 mRNA and protein expression after MPTP. COX-2 mRNA levels are increased by 4 days after MPTP injection (A) compared with controls (C), and almost return to basal levels by 7 days. COX-2 protein contents are minimal in saline-injected mice (sal) (D) but rise in a time-dependent manner after MPTP injection (F). COX-1 expression is not altered by MPTP (A, B, D, and E). Ventral midbrain PGE2 levels are also increased 4 days after MPTP (G). Data are mean ± SEM for four to six mice per group. *, P < 0.05, compared with saline (Newman–Keuls post hoc test).
Figure 2
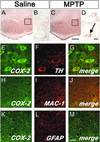
Ventral midbrain illustration of COX-2 immunolocalization. No COX-2-positive cells are seen in saline-injected mice (A and enlarged Inset from A in B). Conversely, COX-2-positive cells are abundant after MPTP (C and enlarged Inset from C in D, arrow). Double immunofluorescence confirms that COX-2 (green) is highly expressed in TH-positive neurons (red; E–G) and not in MAC-1-positive cells (H-J; red) or GFAP-positive cells (K–M; red). [Scale bars, 250 μm (A and C), 10 μm (B and D–G), and 20 μm (H–M).]
Figure 3
Ventral midbrain COX-2 expression is minimal in normal human specimens but is increased 3-fold in PD samples (A). Ventral midbrain PGE2 levels are also increased in PD (B). COX-2 (blue) is not detected in neuromelanized (brown) dopaminergic neurons in controls (C and D) but is well detected in PD (E–G). COX-2 immunostaining (F; arrow) is visible in cells with neuromelanin (F; arrowhead). COX-2 immunostaining is found in the core of a Lewy body (G; arrowhead). Data are mean ± SEM for 3–6 samples for COX-2 protein and 11 samples for PGE2 assessment. *, P < 0.05, compared with normal controls (Newman–Keuls posthoc test). (Scale bar, 25 μm.)
Figure 4
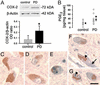
Effect of COX-2 ablation and JNK pathway inhibition on MPTP-induced neuronal loss. TH-positive neuronal counts are shown in Table 1 and appear comparable between saline-injected _Ptgs2_−/− and Ptgs2+/+ mice (A and B and Table 1). SNpc TH-positive neurons are more resistant to MPTP in _Ptgs2_−/− (D) than in Ptgs2+/+ (C) mice, 7 days after MPTP injection. CEP-11004 protects Ptgs2+/+ mice against MPTP neurotoxicity (E). Treatment of _Ptgs2_−/− mice with CEP-11004 does not enhance protection against MPTP (F and Table 1). (G) Ventral midbrain MPTP-induced c-Jun phosphorylation (ρ-c-Jun) inhibition by 1 mg/kg CEP-11004. (H) Ventral midbrain MPTP-induced COX-2 up-regulation is also inhibited by 1 mg/kg CEP-11004. Data are mean ± SEM for three to six mice per group. *, P < 0.05, compared with the other three groups (Newman–Keuls posthoc test). (Scale bar, 250 μm.)
Figure 5
TH-positive neurons and striatal fibers are more resistant to MPTP in mice treated with rofecoxib (25 or 50 mg/kg p.o.; D and F) than in mice receiving vehicle (B), 7 days after MPTP injection (SNpc neuronal counts are shown in G and striatal fiber optical density is shown in H). Rofecoxib by itself has no effect on TH-positive neurons (A, C, and E). Data are mean ± SEM for three to six mice per group. *, P < 0.05, compared with saline-treated controls; #, P < 0.05, compared with rofecoxib-treated MPTP animals (Newman–Keuls posthoc test). (Scale bar, 250 μm.)
Figure 6
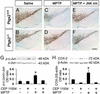
Expression of inflammatory and oxidative stress markers after MPTP. Two days after MPTP injection, mRNA expression of MAC-1 (A and B), ICE (A and C), gp91 (A and D), and iNOS (A and E) are increased in the ventral midbrain and none is attenuated by COX-2 ablation. MAC-1 immunoreactivity is minimal in saline-injected mice in ventral midbrain (F), but is increased after MPTP injection (G; Inset shows MPTP-induced microglial activation at higher magnification). (H) COX-2 inhibition does not attenuate MPTP-induced microglial activation. (I) MPTP increases ventral midbrain protein-bound cysteinyl-dopamine, which is blocked by rofecoxib. Data are mean ± SEM for four to six mice per group. *, P < 0.05, compared with saline treated groups; #, P < 0.05, compared with the other five groups (Newman–Keuls posthoc test). (Scale bar, 250 μm.)
Similar articles
- JNK-mediated induction of cyclooxygenase 2 is required for neurodegeneration in a mouse model of Parkinson's disease.
Hunot S, Vila M, Teismann P, Davis RJ, Hirsch EC, Przedborski S, Rakic P, Flavell RA. Hunot S, et al. Proc Natl Acad Sci U S A. 2004 Jan 13;101(2):665-70. doi: 10.1073/pnas.0307453101. Epub 2004 Jan 2. Proc Natl Acad Sci U S A. 2004. PMID: 14704277 Free PMC article. - COX-2 and neurodegeneration in Parkinson's disease.
Teismann P, Vila M, Choi DK, Tieu K, Wu DC, Jackson-Lewis V, Przedborski S. Teismann P, et al. Ann N Y Acad Sci. 2003 Jun;991:272-7. doi: 10.1111/j.1749-6632.2003.tb07482.x. Ann N Y Acad Sci. 2003. PMID: 12846993 Review. - Response to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) differs in mouse strains and reveals a divergence in JNK signaling and COX-2 induction prior to loss of neurons in the substantia nigra pars compacta.
Boyd JD, Jang H, Shepherd KR, Faherty C, Slack S, Jiao Y, Smeyne RJ. Boyd JD, et al. Brain Res. 2007 Oct 17;1175:107-16. doi: 10.1016/j.brainres.2007.07.067. Epub 2007 Aug 9. Brain Res. 2007. PMID: 17884023 Free PMC article. - JNK inhibitor protects dopaminergic neurons by reducing COX-2 expression in the MPTP mouse model of subacute Parkinson's disease.
Wang Y, Zhang Y, Wei Z, Li H, Zhou H, Zhang Z, Zhang Z. Wang Y, et al. J Neurol Sci. 2009 Oct 15;285(1-2):172-7. doi: 10.1016/j.jns.2009.06.034. Epub 2009 Jul 14. J Neurol Sci. 2009. PMID: 19604516 - Neuroinflammatory processes in Parkinson's disease.
Hunot S, Hirsch EC. Hunot S, et al. Ann Neurol. 2003;53 Suppl 3:S49-58; discussion S58-60. doi: 10.1002/ana.10481. Ann Neurol. 2003. PMID: 12666098 Review.
Cited by
- Neuroprotective Properties of Green Tea (Camellia sinensis) in Parkinson's Disease: A Review.
Malar DS, Prasanth MI, Brimson JM, Sharika R, Sivamaruthi BS, Chaiyasut C, Tencomnao T. Malar DS, et al. Molecules. 2020 Aug 27;25(17):3926. doi: 10.3390/molecules25173926. Molecules. 2020. PMID: 32867388 Free PMC article. Review. - Non-Steroidal Anti-Inflammatory Drugs in Alzheimer's Disease and Parkinson's Disease: Reconsidering the Role of Neuroinflammation.
Moore AH, Bigbee MJ, Boynton GE, Wakeham CM, Rosenheim HM, Staral CJ, Morrissey JL, Hund AK. Moore AH, et al. Pharmaceuticals (Basel). 2010 Jun 2;3(6):1812-1841. doi: 10.3390/ph3061812. Pharmaceuticals (Basel). 2010. PMID: 27713331 Free PMC article. Review. - Serum antibodies from Parkinson's disease patients react with neuronal membrane proteins from a mouse dopaminergic cell line and affect its dopamine expression.
Huber VC, Mondal T, Factor SA, Seegal RF, Lawrence DA. Huber VC, et al. J Neuroinflammation. 2006 Jan 20;3:1. doi: 10.1186/1742-2094-3-1. J Neuroinflammation. 2006. PMID: 16426448 Free PMC article. - Neuroprotective therapy in Parkinson's disease: current status and new directions from experimental and genetic clues.
Lin W, Kang UJ. Lin W, et al. J Clin Neurol. 2005 Oct;1(2):107-20. doi: 10.3988/jcn.2005.1.2.107. Epub 2005 Oct 20. J Clin Neurol. 2005. PMID: 20396458 Free PMC article.
References
- Fahn S, Przedborski S. In: Merritt's Neurology. Rowland L P, editor. Williams & Wilkins, New York: Lippincott; 2000. pp. 679–693.
- Przedborski S, Kostic V, Giladi N, Eidelberg D. In: Dopamine Receptors and Transporters. Sidhu A, Laruelle M, Vernier P, editors. New York: Dekker; 2003. pp. 363–402.
- Wyss-Coray T, Mucke L. Nat Med. 2000;6:973–974. - PubMed
- Almer G, Guegan C, Teismann P, Naini A, Rosoklija G, Hays A P, Chen C, Przedborski S. Ann Neurol. 2001;49:176–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS37345/NS/NINDS NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- NS11766-27A1/NS/NINDS NIH HHS/United States
- NS38370/NS/NINDS NIH HHS/United States
- P01 NS011766/NS/NINDS NIH HHS/United States
- NS38586/NS/NINDS NIH HHS/United States
- R01 NS042269/NS/NINDS NIH HHS/United States
- R01 NS038586/NS/NINDS NIH HHS/United States
- NS42269/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous