Ligand channeling within a G-protein-coupled receptor. The entry and exit of retinals in native opsin - PubMed (original) (raw)
Ligand channeling within a G-protein-coupled receptor. The entry and exit of retinals in native opsin
Sandra A Schädel et al. J Biol Chem. 2003.
Abstract
Deactivation of light-activated rhodopsin (metarhodopsin II) involves, after rhodopsin kinase and arrestin interactions, the hydrolysis of the covalent bond of all-trans-retinal to the apoprotein. Although the long-lived storage form metarhodopsin III is transiently formed, all-trans-retinal is eventually released from the active site. Here we address the question of whether the release results in a retinal that is freely diffusible in the lipid phase of the photoreceptor membrane. The release reaction is accompanied by an increase in intrinsic protein fluorescence (release signal), which arises from the relief of the fluorescence quenching imposed by the retinal in the active site. An analogous fluorescence decrease (uptake signal) was evoked by exogenous retinoids when they non-covalently bound to native opsin membranes. Uptake of 11-cis-retinal was faster than formation of the retinylidene linkage to the apoprotein. Endogenous all-trans-retinal released from the active site during metarhodopsin II decay did not generate the uptake signal. The data show that in addition to the retinylidene pocket (site I) there are two other retinoidbinding sites within opsin. Site II involved in the uptake signal is an entrance site, while the exit site (site III) is occupied when retinal remains bound after its release from site I. Support for a retinal channeling mechanism comes from the rhodopsin crystal structure, which unveiled two putative hydrophobic binding sites. This mechanism enables a unidirectional process for the release of photoisomerized chromophore and the uptake of newly synthesized 11-cis-retinal for the regeneration of rhodopsin.
Figures
Fig. 1
Fluorescence emission spectra of opsin: effect of retinal isomers. All-_trans_-retinal (A) or 11-_cis_-retinal (B) was added to a final concentration of 1 μ
m
to a preparation of photoreceptor disc membranes (1 μ
m
opsin). The fluorescence quenching is seen with both 11-_cis_-retinal and all-_trans_-retinal, indicating the uptake of both isomers into the membrane-bound apoprotein. Spectra C and D are from solubilized membranes (0.1% _n_-dodecyl-β-maltoside). All spectra were recorded at λex = 295 nm, pH 6.5, 20 °C, after 2.5 and 15 min, as indicated.
Fig. 2
Time-dependent fluorescence changes in opsin apoprotein evoked by retinal isomers. Changes in intrinsic protein fluorescence were recorded upon addition of retinals (1 μ
m
) to opsin (1 μ
m
) at 20 °C (pH 6.5). Fluorescence emission of opsin in native membrane (left, a_–_d) and in 0.1% _n_-dodecyl-β-maltoside (right, e_–_h) was recorded at 330 nm with λex = 295 nm. The rapid initial quenching by retinal addition (arrows) is termed the uptake signal. Each sample was illuminated for 15 s with green light (OG 495) after incubation, as indicated by the flash symbol. The fluorescence change after illumination was assigned to the release of the photoisomerized chromophore during decay of the activated metarhodopsin II (8, 9)
Fig. 3
Time-dependent fluorescence changes evoked by retinoids after decay of active metarhodopsin II. Decay of light-activated metarhodopsin II (Meta II, 1st flash) and release of all-_trans_-retinal from the active site caused an increase in the intrinsic protein fluorescence emission (initial slow release signal). Subsequent addition of all-_trans_-retinal (1 μ
m
)(A), 11-_cis_-retinal (1 μ
m
) (B), or all-_trans_-retinol (1 μ
m
) (C and D) to membranes containing the Meta II decay product results in the full reversal of opsin fluorescence to its initial level. The addition of all-_trans_-retinol to opsin apoprotein evokes a uptake signal (C, inset), which is very similar to that obtained after complete Meta II decay (C). Due to rhodopsin regeneration, a second increase in fluorescence is seen for the 11-_cis_-retinal-treated sample (B) upon a second illumination (2nd flash), but not for all-_trans_-retinal- or all-_trans_-retinol-treated samples. The maximal emission intensity was observed upon membrane solubilization with 0.1% dodecyl-β-maltoside (DM) as shown for samples A and B. All-_trans_-retinal release signal is also seen in the presence of NADPH, and subsequent addition of all-_trans_-retinol again leads to a normal uptake signal (D).
Fig. 4
Putative binding sites of retinals on rhodopsin. Two heptane-1,2,3-triol (HTPO)-binding sites were identified in the crystal structure of rhodopsin (15, 16), one site is located close to the palmitoylation site (site II) and the second in the vicinity of C terminus and the 3rd cytoplasmic loop. Additional four HTO molecules are bound to extracellular half of rhodopsin (not shown) (a). The large site II can accommodate all-_trans_-retinal, 11-_cis_-retinal, or both together (b). Site III is located closer to the surface and could be the exit site for all-_trans_-retinal (c). In all figures, the transmembrane segments are colored in light blue and palmitoyl chains in dark blue. Site I is the original binding site for the chromophore, 11-_cis_-retinylidene, which undergoes photoisomerization. All three sites and the side chains of Trp residues are depicted in panel d.
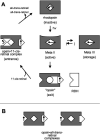
Fig. 5
Intramolecular ligand channeling in rhodopsin. A, in the light-sensitive ground state (rhodopsin, top) the chromophore 11-_cis_-retinal is covalently attached to Lys296 within the binding pocket (site I) of the apoprotein opsin. Photoisomerization to all-_trans_-retinal results in formation of the active intermediate metarhodopsin II (Meta II, center). Meta II decays to a storage form (Meta III, right) or into the apoprotein opsin with the photolyzed all-_trans_-retinal bound in the exit site (site III; opsin, bottom). In this state, the retinal does not quench intrinsic Trp fluorescence. Opsin eventually returns to the ground state via a transiently formed opsin·11-_cis_-retinal·complex, which contains both retinal isomers bound to the entrance (site II) and exit (site III). RDH has access to its retinal substrate while bound to the exit site of opsin. Opsin·all-_trans_-retinal complexes (B) are formed with the stripped apoprotein (right) or the Meta II decay product (left) in the presence of free diffusible all-_trans_-retinal.
Similar articles
- Secondary binding sites of retinoids in opsin: characterization and role in regeneration.
Heck M, Schädel SA, Maretzki D, Hofmann KP. Heck M, et al. Vision Res. 2003 Dec;43(28):3003-10. doi: 10.1016/j.visres.2003.08.011. Vision Res. 2003. PMID: 14611936 - Crystal structure of the ligand-free G-protein-coupled receptor opsin.
Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Park JH, et al. Nature. 2008 Jul 10;454(7201):183-7. doi: 10.1038/nature07063. Epub 2008 Jun 18. Nature. 2008. PMID: 18563085 - Signaling states of rhodopsin. Formation of the storage form, metarhodopsin III, from active metarhodopsin II.
Heck M, Schädel SA, Maretzki D, Bartl FJ, Ritter E, Palczewski K, Hofmann KP. Heck M, et al. J Biol Chem. 2003 Jan 31;278(5):3162-9. doi: 10.1074/jbc.M209675200. Epub 2002 Nov 9. J Biol Chem. 2003. PMID: 12427735 Free PMC article. - Photophysiological functions of visual pigments.
Yoshizawa T. Yoshizawa T. Adv Biophys. 1984;17:5-67. doi: 10.1016/0065-227x(84)90024-8. Adv Biophys. 1984. PMID: 6242325 Review. - Retinal dynamics during light activation of rhodopsin revealed by solid-state NMR spectroscopy.
Brown MF, Salgado GF, Struts AV. Brown MF, et al. Biochim Biophys Acta. 2010 Feb;1798(2):177-93. doi: 10.1016/j.bbamem.2009.08.013. Epub 2009 Aug 28. Biochim Biophys Acta. 2010. PMID: 19716801 Free PMC article. Review.
Cited by
- Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo.
Maeda A, Maeda T, Imanishi Y, Kuksa V, Alekseev A, Bronson JD, Zhang H, Zhu L, Sun W, Saperstein DA, Rieke F, Baehr W, Palczewski K. Maeda A, et al. J Biol Chem. 2005 May 13;280(19):18822-32. doi: 10.1074/jbc.M501757200. Epub 2005 Mar 8. J Biol Chem. 2005. PMID: 15755727 Free PMC article. - A rhodopsin exhibiting binding ability to agonist all-trans-retinal.
Tsukamoto H, Terakita A, Shichida Y. Tsukamoto H, et al. Proc Natl Acad Sci U S A. 2005 May 3;102(18):6303-8. doi: 10.1073/pnas.0500378102. Epub 2005 Apr 25. Proc Natl Acad Sci U S A. 2005. PMID: 15851682 Free PMC article. - Functional characterization of rhodopsin monomers and dimers in detergents.
Jastrzebska B, Maeda T, Zhu L, Fotiadis D, Filipek S, Engel A, Stenkamp RE, Palczewski K. Jastrzebska B, et al. J Biol Chem. 2004 Dec 24;279(52):54663-75. doi: 10.1074/jbc.M408691200. Epub 2004 Oct 15. J Biol Chem. 2004. PMID: 15489507 Free PMC article. - Matrices for Sensors from Inorganic, Organic, and Biological Nanocomposites.
Nicolini C, Sivozhelezov V, Bavastrello V, Bezzerra T, Scudieri D, Spera R, Pechkova E. Nicolini C, et al. Materials (Basel). 2011 Aug 24;4(8):1483-1518. doi: 10.3390/ma4081483. Materials (Basel). 2011. PMID: 28824154 Free PMC article. Review. - Phospholipid meets all-trans-retinal: the making of RPE bisretinoids.
Sparrow JR, Wu Y, Kim CY, Zhou J. Sparrow JR, et al. J Lipid Res. 2010 Feb;51(2):247-61. doi: 10.1194/jlr.R000687. Epub 2009 Aug 7. J Lipid Res. 2010. PMID: 19666736 Free PMC article. Review.
References
- McBee JK, Palczewski K, Baehr W, Pepperberg DR. Prog. Retin. Eye Res. 2001;20:469–529. - PubMed
- Okada T, Ernst OP, Palczewski K, Hofmann KP. Trends Biochem. Sci. 2001;26:318–324. - PubMed
- Hofmann KP. Novartis Found Symp. 1999;224:158–175. discussion 175–180. - PubMed
- DeGrip WJ, DeLange F, Klaassen CH, Verdegem PJ, Wallace-Williams S, Creemers AF, Bergo V, Bovee PH, Raap J, Rothschild KJ, DeGroot HJ, Lugtenburg J. Novartis Found Symp. 1999;224:102–118. discussion 118–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources