Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability - PubMed (original) (raw)
Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability
Heike Hering et al. J Neurosci. 2003.
Abstract
Cholesterol/sphingolipid microdomains (lipid rafts) in the membrane are involved in protein trafficking, formation of signaling complexes, and regulation of actin cytoskeleton. Here, we show that lipid rafts exist abundantly in dendrites of cultured hippocampal neurons, in which they are associated with several postsynaptic proteins including surface AMPA receptors. Depletion of cholesterol/sphingolipid leads to instability of surface AMPA receptors and gradual loss of synapses (both inhibitory and excitatory) and dendritic spines. The remaining synapses and spines in raft-depleted neurons become greatly enlarged. The importance of lipid rafts for normal synapse density and morphology could explain why cholesterol promotes synapse maturation in retinal ganglion cells (Mauch et al., 2001) and offers a potential link between disordered cholesterol metabolism and the synapse loss seen in neurodegenerative disease.
Figures
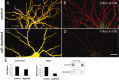
Fig. 1.
Postsynaptic proteins associated with lipid rafts.A, Adult rat brain membranes were extracted in 0.5% Triton X-100 and separated on a density gradient formed from 30, 25, and 5% Nycodenz. Thirteen fractions (from top to bottom of gradient) were immunoblotted for the indicated proteins. The fractions were also assayed for sphingolipid (dot-blot assay using cholera-toxin subunit B), cholesterol, and total protein (see Materials and Methods).B, The same Triton X-100 extract used in_A_ was separated on a density gradient formed from 30, 25, 15, and 5% of Nycodenz. Nine fractions were collected from the top and immunoblotted for the indicated proteins. TfR, Transferrin receptor; Chol. tox., cholera-toxin subunit B.
Fig. 2.
Detergent-resistant membranes in dendrites of cultured neurons. A, Hippocampal neuron at 21 DIV double-labeled with DiIC18 (red) and FAST-DiO (green).B, DiIC18/FAST-DiO-labeled hippocampal neuron after extraction with 0.5% Triton X-100 at 4°C.C, Hippocampal neuron treated with mevastatin, fumonisin B1, and mevalonate for 5 d labeled with DiIC18 and FAST-DiO. D, DiIC18/FAST-DiO-labeled hippocampal neuron treated as in C, after extraction with 0.5% Triton X-100 at 4°C. Scale bar, 20 μm. E, Cholesterol and sphingolipid levels in control and raft-depleted cultures. Cholesterol levels in raft-depleted cultures were decreased ∼31% (p < 0.05) on the basis of an enzymatic cholesterol-detection assay, or ∼72% (p < 0.001) on the basis of binding of fluorescent filipin. Ganglioside GM1 levels were reduced on the basis of cholera-toxin subunit B (chol. tox.) binding in a dot-blot assay.
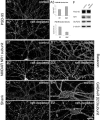
Fig. 3.
Lipid-raft depletion reduces the number but increases the size of synapses. All images were taken from hippocampal neurons at 21 DIV. A1, Control hippocampal neurons stained for PSD-95. A2, Hippocampal neurons treated for 7 d with mevastatin, fumonisin B1, and mevalonate (raft-depleted) and stained for PSD-95. A3, Quantitation of PSD-95 cluster size and density in control (ctrl) and raft-depleted (depl) neurons. Histograms show mean ± SEM;n = 5 microscope fields for each condition. Differences in cluster size (p = 0.0036) and density (p = 0.0024) are significant (Student's_t_ test). B–E, Control and raft-depleted neurons stained for NR1 subunit of NMDA receptor (NMDAR;B1, B2), Shank (C1,C2), bassoon (D1, D2), and β2/3 subunit of GABAA receptor (GABAAR; E1,E2). Scale bar, 20 μm. F, Total cell lysate of control and raft-depleted hippocampal cultures immunoblotted for the indicated proteins. Anti-tubulin immunoblotting confirmed equal loading of protein.
Fig. 4.
Raft depletion reduces the number and increases the size of dendritic spines. A, Dendrites of control hippocampal neuron at 19 DIV. B, Neuron at 19 DIV raft depleted for 5 d. Arrowheads indicate enlarged spines.C, Dendrites from a neuron at 24 DIV raft depleted for 10 d. Dendritic morphology of hippocampal neurons was visualized by staining for transfected PSD-95. D, Cumulative distribution of spine width and length in control and raft-depleted neurons. Spine length, 1.75 ± 0.79 μm (mean ± SD) for controls and 1.91 ± 0.76 μm for raft-depleted neurons (p = 0.01; Mann–Whitney U test). Spine width, 1 ± 0.31 μm (mean ± SD) for control; 1.28 ± 0.47 μm for raft-depleted neurons (p< 0.0001; Mann–Whitney U test). E,F, Dendrite from a control neuron (ctrl;E) and a raft-depleted neuron (depl;F), double-stained for transfected PSD-95 (red) and endogenous bassoon (green). G, H, FM 1-43 labeling of control (G) and raft-depleted (H) neurons. Scale bars:A_–_D, 20 μm;_E-_-H, 10 μm.
Fig. 5.
Effects of acute cholesterol extraction by methyl-β-cyclodextrin on spines. A–E, Dendritic morphology was visualized by DiI staining of hippocampal neurons at 25 DIV: untreated control neuron (A), neuron extracted with 5 m
m
mβCD (B), neuron extracted with 5 m
m
mβCD in the presence of 100 μ
m
AP-5 and 30 μ
m
CNQX (C), neuron treated with 2 μ
m
jasplakinolide (jasp; D, E), or neuron treated with 2 μ
m
jasplakinolide followed by mβCD (E). F, G, Dendrite of control neuron at 25 DIV (F) or an mβCD-extracted neuron (G) labeled for F-actin with Oregon Green–phalloidin. Scale bars:A_–_D, 20 μm; F,G, 10 μm
Fig. 6.
Raft association of surface AMPA receptors.A1, A2, Surface AMPA receptors on hippocampal neurons at 19 DIV revealed by labeling live neurons with antibodies against extracellular domain of GluR1. B1,B2, Surface AMPA receptors after extraction of neurons with 0.5% Triton X-100 (Tx-100) at 4°C. (A2 and_B2_ are higher magnifications of dendrites than_A1_ and B1.) C1, Surface AMPA receptors after extraction with 0.5% Triton X-100 at 37°C. D1, Surface AMPA receptors after extraction with 0.5% saponin (sap), followed by 0.5% Triton X-100 at 4°C. (C2 and D2 show the same neuron as in_C1_ and D1, respectively, double-labeled with MAP2 antibody to indicate dendrites). E, Double-labeling of PSD-95 (E1) and surface AMPA receptors (E2) in untreated control neurons.F, Double-labeling of PSD-95 (F1) and surface AMPA receptors (F2) in neurons extracted with 0.5% Triton X-100 at 4°C. G, Double-labeling of PSD-95 (G1) and surface AMPA receptors (G2) in neurons extracted with 0.5% saponin (sap), followed by 0.5% Triton X-100 at 4°C. Bottom panels (E3, F3, G3) show in color the merged image of PSD-95 (red) and AMPA-receptor surface staining (green). Scale bars: A1, B1,C_–_G, 20 μm; A2,B2, 5 μm.
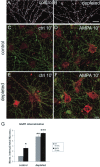
Fig. 7.
AMPA receptor internalization in raft-depleted neurons. A, B, Surface AMPA receptors (GluR1) on control (A) and raft-depleted (B) hippocampal neurons. C–F, Antibody-feeding assay showing remaining surface AMPA receptors (green) and internalized AMPA receptors (red) at 10 min after surface labeling of AMPA receptors on live neurons. C, D, Non-raft-depleted neurons, untreated (C) or stimulated with 100 μ
m
AMPA in the presence of 100 μ
m
AP-5 (D). E, F, Raft-depleted neurons, untreated (E) or stimulated with 100 μ
m
AMPA and 100 μ
m
AP-5 (F). G, Quantitation of results illustrated in C_–_F. Histograms show mean ± SEM; n = 6 microscope fields for each condition. *p = 0.035 and **p = 0.0012 compared with control (ctrl) 10′; ***p = 0.0012 compared with control/AMPA 10′ (Mann–Whitney _U_test). Scale bars: A, B, 20 μm;C_–_F, 10 μm.
Similar articles
- Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors.
Allison DW, Gelfand VI, Spector I, Craig AM. Allison DW, et al. J Neurosci. 1998 Apr 1;18(7):2423-36. doi: 10.1523/JNEUROSCI.18-07-02423.1998. J Neurosci. 1998. PMID: 9502803 Free PMC article. - Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting.
Ko J, Kim S, Valtschanoff JG, Shin H, Lee JR, Sheng M, Premont RT, Weinberg RJ, Kim E. Ko J, et al. J Neurosci. 2003 Mar 1;23(5):1667-77. doi: 10.1523/JNEUROSCI.23-05-01667.2003. J Neurosci. 2003. PMID: 12629171 Free PMC article. - Regulation of AMPA receptor localization in lipid rafts.
Hou Q, Huang Y, Amato S, Snyder SH, Huganir RL, Man HY. Hou Q, et al. Mol Cell Neurosci. 2008 Jun;38(2):213-23. doi: 10.1016/j.mcn.2008.02.010. Epub 2008 Mar 5. Mol Cell Neurosci. 2008. PMID: 18411055 Free PMC article. - Role of syntaxin 4 in activity-dependent exocytosis and synaptic plasticity in hippocampal neurons.
Mohanasundaram P, Shanmugam MM. Mohanasundaram P, et al. Sci Signal. 2010 Oct 19;3(144):jc7. doi: 10.1126/scisignal.3144jc7. Sci Signal. 2010. PMID: 20959521 Review. - The state of lipid rafts: from model membranes to cells.
Edidin M. Edidin M. Annu Rev Biophys Biomol Struct. 2003;32:257-83. doi: 10.1146/annurev.biophys.32.110601.142439. Epub 2003 Jan 16. Annu Rev Biophys Biomol Struct. 2003. PMID: 12543707 Review.
Cited by
- Association between tetrodotoxin resistant channels and lipid rafts regulates sensory neuron excitability.
Pristerà A, Baker MD, Okuse K. Pristerà A, et al. PLoS One. 2012;7(8):e40079. doi: 10.1371/journal.pone.0040079. Epub 2012 Aug 1. PLoS One. 2012. PMID: 22870192 Free PMC article. - Brain Cholesterol Metabolism and Its Defects: Linkage to Neurodegenerative Diseases and Synaptic Dysfunction.
Petrov AM, Kasimov MR, Zefirov AL. Petrov AM, et al. Acta Naturae. 2016 Jan-Mar;8(1):58-73. Acta Naturae. 2016. PMID: 27099785 Free PMC article. - Remembering Mechanosensitivity of NMDA Receptors.
Johnson LR, Battle AR, Martinac B. Johnson LR, et al. Front Cell Neurosci. 2019 Dec 5;13:533. doi: 10.3389/fncel.2019.00533. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31866826 Free PMC article. Review. - Elastic properties of the cell surface and trafficking of single AMPA receptors in living hippocampal neurons.
Yersin A, Hirling H, Kasas S, Roduit C, Kulangara K, Dietler G, Lafont F, Catsicas S, Steiner P. Yersin A, et al. Biophys J. 2007 Jun 15;92(12):4482-9. doi: 10.1529/biophysj.106.092742. Epub 2007 Mar 30. Biophys J. 2007. PMID: 17400692 Free PMC article. - Association of lipid rafts cholesterol with clinical profile in fragile X syndrome.
Toupin A, Benachenhou S, Abolghasemi A, Laroui A, Galarneau L, Fülöp T, Corbin F, Çaku A. Toupin A, et al. Sci Rep. 2022 Feb 21;12(1):2936. doi: 10.1038/s41598-022-07064-z. Sci Rep. 2022. PMID: 35190617 Free PMC article.
References
- Becher A, White JH, McIlhinney RA. The gamma-aminobutyric acid receptor B, but not the metabotropic glutamate receptor type-1, associates with lipid rafts in the rat cerebellum. J Neurochem. 2001;79:787–795. - PubMed
- Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–114. - PubMed
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
- Bruckner K, Pablo Labrador J, Scheiffele P, Herb A, Seeburg PH, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron. 1999;22:511–524. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous