Intravitreal gene therapy reduces lysosomal storage in specific areas of the CNS in mucopolysaccharidosis VII mice - PubMed (original) (raw)
Intravitreal gene therapy reduces lysosomal storage in specific areas of the CNS in mucopolysaccharidosis VII mice
Anne K Hennig et al. J Neurosci. 2003.
Abstract
The mucopolysaccharidoses (MPSs) are lysosomal storage diseases resulting from impaired catabolism of sulfated glycosaminoglycans. MPS VII mice lack lysosomal beta-glucuronidase (GUSB) activity, leading to the accumulation of partially degraded chondroitin, dermatan, and heparan sulfates in most tissues. Consequently, these mice develop most of the symptoms exhibited by human MPS VII patients, including progressive visual and cognitive deficits. To investigate the effects of reducing lysosomal storage in nervous tissues, we injected recombinant adeno-associated virus encoding GUSB directly into the vitreous humor of young adult mice. Interestingly, GUSB activity was subsequently detected in the brains of the recipients. At 8-12 weeks after treatment, increased GUSB activity and reduced lysosomal distension were found in regions of the thalamus and tectum that received inputs from the injected eye. Lysosomal storage was also reduced in adjacent nonvisual regions, including the hippocampus, as well as in the visual cortex. The findings suggest that both diffusion and trans-synaptic transfer contribute to the dissemination of enzyme activity within the CNS. Intravitreal injection may thus provide a means of delivering certain therapeutic gene products to specific areas within the CNS.
Figures
Fig. 1.
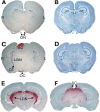
GUSB activity is present in the brain along the visual pathway. A, An oblique coronal section through the brain of an MPS VII mouse that received a unilateral intravitreal injection of AAVβGEnh. GUSB activity (red) is present in the optic nerve (ON) from the mouse's left (injected) eye but not in the ON from the untreated right eye. B, An adjacent section stained with cresyl violet shows the cellular architecture. C, A parallel section through the optic chiasm (OC) and tract (OT), lateral geniculate nucleus (LGN), and superior colliculus (SC) of the same brain shows prominent GUSB activity on the side contralateral to the injected eye, as well as a lower level of activity in the ipsilateral LGN. This pattern of enzyme activity was seen in all brains from MPS VII mice that received unilateral intravitreal AAVβGEnh.D, A section adjacent to that in C, stained with cresyl violet. E, A coronal section through the brain of an MPS VII mouse that received bilateral intravitreal AAVβGEnh shows a symmetrical pattern of high-level enzyme activity.F, A parallel section from the same brain as_E_ shows high-level activity in both SCs. L, Mouse's left; R, mouse's right. Sections stained for GUSB activity are counterstained with methyl green.
Fig. 2.
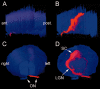
The distribution of GUSB activity throughout the brain is seen in a three-dimensional reconstruction from another 16-week-old MPS VII mouse treated unilaterally with intravitreal AAVβGEnh at 4 weeks of age. A, Lateral view of the reconstructed image shows surface contours of the cerebral hemisphere, cerebellum, and brainstem, from anterior (ant.) to posterior (post.).B, Rendering the image transparent shows the pattern of GUSB activity along the visual pathway. C, Frontal view, showing the surface contours of the reconstruction. The right (blue) and left (red) optic nerves (ON) can be seen entering the brain. Right and left indicate the right and left sides of the mouse.D, In the transparent image, GUSB activity is most prominent in the optic nerve and tract, lateral geniculate nucleus (LGN), and superior colliculus (SC) on the right side, contralateral to the injected eye. Lower levels of activity can also be seen in the ipsilateral optic pathway.
Fig. 3.
Intravitreal AAVβGEnh decreases lysosomal storage in hippocampus and visual cortex as well as LGN. Pairs of toluidine blue-stained, 0.5-μm-thick sections from the CA1 and CA4 regions of the hippocampus, visual cortex (VC), nonvisual cortex (NVC), and lateral geniculate nucleus (LGN) are from two 16-week-old MPS VII mice. The sections indicated by “−” are from the mouse that received no treatment. The sections indicated by “+” are from a littermate that received unilateral intravitreal AAVβGEnh at 4 weeks of age. Black arrows indicate distended vacuoles in neurons; white arrows indicate storage in microglia and other nonneuronal cells. The approximate sites of the respective histologic samples are indicated on the GUSB-stained cryostat section.
Fig. 4.
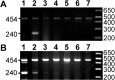
PCR reveals AAVβGEnh DNA in the injected eyes of treated mice, but not in brain samples. A, DNA from the right optic nerve (lane 1), left (injected) eye (lane 2), left optic nerve (lane 3), optic chiasm (lane 4), right LGN (lane 5), posterior LGN + anterior SC (lane 6), and SC (lane 7) of a 14-week-old MPS VII mouse that received an intravitreal injection at 6 weeks of age. Primers amplified a fragment of 240 bp from the human GUSB cDNA sequence encoded by AAVβGEnh as well as a 454 bp fragment from the endogenous mouse GUSB gene. B, Murine fibroblasts (3521 cells) containing one retroviral hGUSB cDNA insert per cell (lane 1) were titered with untransduced 3521 cells (lane 7). Cell mixtures contained one copy of hGUSB per 100 haploid genomes (lane 2), one per 1 × 103 (lane 3), one per 2 × 103 (lane 4), one per 104 (lane 5), and one per 2 × 104 (lane 6). Fragment sizes are given in base pairs.
Similar articles
- Recombinant adeno-associated virus-mediated correction of lysosomal storage within the central nervous system of the adult mucopolysaccharidosis type VII mouse.
Sferra TJ, Qu G, McNeely D, Rennard R, Clark KR, Lo WD, Johnson PR. Sferra TJ, et al. Hum Gene Ther. 2000 Mar 1;11(4):507-19. doi: 10.1089/10430340050015707. Hum Gene Ther. 2000. PMID: 10724030 - Intracranial injection of recombinant adeno-associated virus improves cognitive function in a murine model of mucopolysaccharidosis type VII.
Frisella WA, O'Connor LH, Vogler CA, Roberts M, Walkley S, Levy B, Daly TM, Sands MS. Frisella WA, et al. Mol Ther. 2001 Mar;3(3):351-8. doi: 10.1006/mthe.2001.0274. Mol Ther. 2001. PMID: 11273777 - AAV-mediated intravitreal gene therapy reduces lysosomal storage in the retinal pigmented epithelium and improves retinal function in adult MPS VII mice.
Hennig AK, Ogilvie JM, Ohlemiller KK, Timmers AM, Hauswirth WW, Sands MS. Hennig AK, et al. Mol Ther. 2004 Jul;10(1):106-16. doi: 10.1016/j.ymthe.2004.03.018. Mol Ther. 2004. PMID: 15233947 - Murine mucopolysaccharidosis type VII: the impact of therapies on the clinical course and pathology in a murine model of lysosomal storage disease.
Vogler C, Sands MS, Galvin N, Levy B, Thorpe C, Barker J, Sly WS. Vogler C, et al. J Inherit Metab Dis. 1998 Aug;21(5):575-86. doi: 10.1023/a:1005423222927. J Inherit Metab Dis. 1998. PMID: 9728337 Review. - Murine mucopolysaccharidosis VIL: impact of therapies on the phenotype, clinical course, and pathology in a model of a lysosomal storage disease.
Vogler C, Barker J, Sands MS, Levy B, Galvin N, Sly WS. Vogler C, et al. Pediatr Dev Pathol. 2001 Sep-Oct;4(5):421-33. doi: 10.1007/s10024001-0079-1. Pediatr Dev Pathol. 2001. PMID: 11779044 Review.
Cited by
- Evaluation of neuroretina following i.v. or intra-CSF AAV9 gene replacement in mice with MPS IIIA, a childhood dementia.
Beard H, Winner L, Shoubridge A, Parkinson-Lawrence E, Lau AA, Mubarokah SN, Lance TR, King B, Scott W, Snel MF, Trim PJ, Hemsley KM. Beard H, et al. CNS Neurosci Ther. 2024 Aug;30(8):e14919. doi: 10.1111/cns.14919. CNS Neurosci Ther. 2024. PMID: 39123298 Free PMC article. - Visual System Impairment in a Mouse Model of Krabbe Disease: The Twitcher Mouse.
Tonazzini I, Cerri C, Del Grosso A, Antonini S, Allegra M, Caleo M, Cecchini M. Tonazzini I, et al. Biomolecules. 2020 Dec 23;11(1):7. doi: 10.3390/biom11010007. Biomolecules. 2020. PMID: 33374753 Free PMC article. - Long-term AAV vector gene and protein expression in mouse brain from a small pan-cellular promoter is similar to neural cell promoters.
Husain T, Passini MA, Parente MK, Fraser NW, Wolfe JH. Husain T, et al. Gene Ther. 2009 Jul;16(7):927-32. doi: 10.1038/gt.2009.52. Epub 2009 May 21. Gene Ther. 2009. PMID: 19458648 Free PMC article. - The taming of the cell penetrating domain of the HIV Tat: myths and realities.
Chauhan A, Tikoo A, Kapur AK, Singh M. Chauhan A, et al. J Control Release. 2007 Feb 12;117(2):148-62. doi: 10.1016/j.jconrel.2006.10.031. Epub 2006 Nov 17. J Control Release. 2007. PMID: 17196289 Free PMC article. Review.
References
- Bosch A, Ferret E, Desmaris N, Trono D, Heard JM. Reversal of pathology in the entire brain of mucopolysaccharidosis type VII mice after lentivirus-mediated gene transfer. Hum Gene Ther. 2000;11:1139–1150. - PubMed
- Brooks AI, Stein CS, Hughes SM, Heth J, McCray PM, Jr, Sauter SL, Johnston JC, Cory-Slechta DA, Federoff HJ, Davidson BL. Functional correction of established central nervous system deficits in an animal model of lysosomal storage disease with feline immunodeficiency virus-based vectors. Proc Natl Acad Sci USA. 2002;99:6216–6221. - PMC - PubMed
- Daly T, Okuyama T, Vogler C, Haskins M, Muzyczka N, Sands MS. Neonatal intramuscular injection with recombinant adeno-associated virus results in prolonged β-glucuronidase expression in situ and correction of liver pathology in mucopolysaccharidosis type VII mice. Hum Gene Ther. 1999a;10:85–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY012260/EY/NEI NIH HHS/United States
- NS 044520/NS/NINDS NIH HHS/United States
- P30 DC004665/DC/NIDCD NIH HHS/United States
- P30 EY002687/EY/NEI NIH HHS/United States
- R01 DK057586/DK/NIDDK NIH HHS/United States
- DK 57586/DK/NIDDK NIH HHS/United States
- DC 04665/DC/NIDCD NIH HHS/United States
- 1R03 DC04946-01/DC/NIDCD NIH HHS/United States
- EY 12260/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical