Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine - PubMed (original) (raw)
Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine
Venkata S Mattay et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Monamines subserve many critical roles in the brain, and monoaminergic drugs such as amphetamine have a long history in the treatment of neuropsychiatric disorders and also as a substance of abuse. The clinical effects of amphetamine are quite variable, from positive effects on mood and cognition in some individuals, to negative responses in others, perhaps related to individual variations in monaminergic function and monoamine system genes. We explored the effect of a functional polymorphism (val(158)-met) in the catechol O-methyltransferase gene, which has been shown to modulate prefrontal dopamine in animals and prefrontal cortical function in humans, on the modulatory actions of amphetamine on the prefrontal cortex. Amphetamine enhanced the efficiency of prefrontal cortex function assayed with functional MRI during a working memory task in subjects with the high enzyme activity val/val genotype, who presumably have relatively less prefrontal synaptic dopamine, at all levels of task difficulty. In contrast, in subjects with the low activity met/met genotype who tend to have superior baseline prefrontal function, the drug had no effect on cortical efficiency at low-to-moderate working memory load and caused deterioration at high working memory load. These data illustrate an application of functional neuroimaging in pharmacogenomics and extend basic evidence of an inverted-"U" functional-response curve to increasing dopamine signaling in the prefrontal cortex. Further, individuals with the met/met catechol O-methyltransferase genotype appear to be at increased risk for an adverse response to amphetamine.
Figures
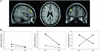
Figure 1
(A) Representative sagittal, axial, and coronal slices from a group analysis showing locales of a drug × COMT genotype interaction in the left PFC (Brodmann's area 9/46, Talaraich coordinates −38, 45, and 24, Z score >2.3, P < 0.01, corrected) during the WM task (see Fig. 5, which is published as supporting information on the PNAS web site). (_B_) Percent change in BOLD signal in the left PFC during the N-back relative to the control task on AMP and PBO conditions. The percent change in BOLD signal was calculated post hoc by using the mean signal intensity values extracted from the cluster of voxels that showed a significant drug × COMT genotype interaction (see text for details). Of note, similar to the findings of Egan _et al._ (19), there is a main effect of genotype at baseline (PBO condition), as _val_/_val_ (●) individuals are less efficient than _met_/_met_ individuals (♦) at all levels of task difficulty. Other PFC areas that were significant on this interaction analysis include Brodmann's area 6 (8, −1, and 55, _Z_ score >3, P < 0.001) and Brodmann's area 44 (−49, 11, and 10, _Z_ score >2, P < 0.02). There were no areas outside the PFC that showed a significant drug × genotype interaction.
Figure 2
N-back performance based on genotype and drug condition. *, AMP caused a significant decrease in performance on the 3-back task in met/met individuals (P < 0.05; Duncan post hoc test). There was no significant difference in serum AMP levels across the three genotypes (mean ± SEM; val/val = 54 ± 3.6 ng/ml, val/met = 53 ± 2.9 ng/ml, and met/met = 56 ± 2.7 ng/ml; ANOVA F(2,22) = 0.27, P = 0.8) and no effect of genotype on any of the clinical variables. Further details on clinical variables are available in Supporting Text.
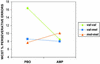
Figure 3
WCST percent perseverative errors on AMP and PBO showing a significant drug × genotype interaction (matched groups; ANOVA F(2,14) = 5.2, P < 0.02). Note that individuals with the val/val genotype perform better on AMP (fewer errors), whereas individuals the met/met genotype get worse (more errors) and individuals with the val/met genotype show no discernable effect on performance. Analysis of data from all subjects (val/val = 10, val/met = 10, and met/met = 6) who performed the task revealed a similar drug × genotype interaction (ANOVA F(2,23) = 3.7, P < 0.04). In addition, analysis using percent total errors (perseverative and nonperseverative errors; a measure of general performance) also revealed a significant drug × genotype interaction (ANOVA F(2,22) = 4.3, P < 0.02). Subjects with the val/val genotype showed better overall performance scores (i.e., percent fewer total errors) on AMP, whereas subjects with the met/met genotype showed the opposite response. In essence, this suggests that, although the val/val subjects on PBO made more total errors than they did on AMP, and met/met subjects on AMP made more total errors than on PBO, perseverative errors made up a major portion of the total errors. Thus, AMP did not induce perseverative errors independent of genotype.
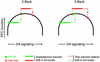
Figure 4
Theoretical inverted-U model describing the effects of COMT genotype, WM load, and AMP on PFC DA signaling and function. The model has three simplified assumptions: (i) fixed baseline positions for each genotype group based on differential COMT activity; (ii) greater DA release with increasing WM load; and (iii) a fixed pharmacological effect of AMP on increasing synaptic DA levels. Thus, at baseline, individuals homozygous for the val allele (who have relatively poorer prefrontal function, greater COMT activity, and presumably less DA) are located on the up slope of the normal range, whereas individuals homozygous for the met allele are located near the peak. In val/val individuals, AMP improves PFC function as DA signaling is shifted to more optimal levels at all load conditions. In contrast, in individuals homozygous for the met allele, AMP shifts DA levels onto the down slope of the inverted-U curve, which has no effect, or a deleterious effect, depending on the magnitude of additional shifts in DA levels associated with increasing processing demands. (See text for further discussion.) The model predicts that higher doses of AMP would also compromise prefrontal function in individuals with the val/val genotype.
Similar articles
- Variation in catechol-o-methyltransferase val158 met genotype associated with schizotypy but not cognition: a population study in 543 young men.
Stefanis NC, Van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, Stefanis CN. Stefanis NC, et al. Biol Psychiatry. 2004 Oct 1;56(7):510-5. doi: 10.1016/j.biopsych.2004.06.038. Biol Psychiatry. 2004. PMID: 15450787 Clinical Trial. - The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks.
Heinz A, Smolka MN. Heinz A, et al. Rev Neurosci. 2006;17(3):359-67. doi: 10.1515/revneuro.2006.17.3.359. Rev Neurosci. 2006. PMID: 16878403 Review. - Impact of catechol-O-methyltransferase Val(108/158) Met genotype on hippocampal and prefrontal gray matter volume.
Cerasa A, Gioia MC, Labate A, Liguori M, Lanza P, Quattrone A. Cerasa A, et al. Neuroreport. 2008 Mar 5;19(4):405-8. doi: 10.1097/WNR.0b013e3282f5f784. Neuroreport. 2008. PMID: 18287936 - Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism.
Giakoumaki SG, Roussos P, Bitsios P. Giakoumaki SG, et al. Neuropsychopharmacology. 2008 Dec;33(13):3058-68. doi: 10.1038/npp.2008.82. Epub 2008 Jun 4. Neuropsychopharmacology. 2008. PMID: 18536698 Clinical Trial. - Catechol-O-methyltransferase, dopamine, and sleep-wake regulation.
Dauvilliers Y, Tafti M, Landolt HP. Dauvilliers Y, et al. Sleep Med Rev. 2015 Aug;22:47-53. doi: 10.1016/j.smrv.2014.10.006. Epub 2014 Oct 27. Sleep Med Rev. 2015. PMID: 25466290 Review.
Cited by
- Selective overexpression of Comt in prefrontal cortex rescues schizophrenia-like phenotypes in a mouse model of 22q11 deletion syndrome.
Kimoto S, Muraki K, Toritsuka M, Mugikura S, Kajiwara K, Kishimoto T, Illingworth E, Tanigaki K. Kimoto S, et al. Transl Psychiatry. 2012 Aug 7;2(8):e146. doi: 10.1038/tp.2012.70. Transl Psychiatry. 2012. PMID: 22872161 Free PMC article. - Moment-to-moment brain signal variability: a next frontier in human brain mapping?
Garrett DD, Samanez-Larkin GR, MacDonald SW, Lindenberger U, McIntosh AR, Grady CL. Garrett DD, et al. Neurosci Biobehav Rev. 2013 May;37(4):610-24. doi: 10.1016/j.neubiorev.2013.02.015. Epub 2013 Mar 1. Neurosci Biobehav Rev. 2013. PMID: 23458776 Free PMC article. Review. - Bad habits-good goals? Meta-analysis and translation of the habit construct to alcoholism.
Giannone F, Ebrahimi C, Endrass T, Hansson AC, Schlagenhauf F, Sommer WH. Giannone F, et al. Transl Psychiatry. 2024 Jul 19;14(1):298. doi: 10.1038/s41398-024-02965-1. Transl Psychiatry. 2024. PMID: 39030169 Free PMC article. Review. - Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors.
Apud JA, Weinberger DR. Apud JA, et al. CNS Drugs. 2007;21(7):535-57. doi: 10.2165/00023210-200721070-00002. CNS Drugs. 2007. PMID: 17579498 Review. - Association between the catechol-O-methyltransferase Val158Met polymorphism and self-perceived social acceptance in adolescent girls.
Waugh CE, Dearing KF, Joormann J, Gotlib IH. Waugh CE, et al. J Child Adolesc Psychopharmacol. 2009 Aug;19(4):395-401. doi: 10.1089/cap.2008.0141. J Child Adolesc Psychopharmacol. 2009. PMID: 19702491 Free PMC article.
References
- Sprague R L, Sleator E K. Science. 1977;198:1274–1276. - PubMed
- Robbins T. Exp Brain Res. 2000;133:130–138. - PubMed
- Kimberg D Y, D'Esposito M, Farah M J. NeuroReport. 1997;8:3581–3585. - PubMed
- Mattay V S, Callicott J H, Bertolino A, Heaton I, Frank J A, Coppola R, Berman K F, Goldberg T E, Weinberger D R. NeuroImage. 2000;12:268–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous