SHED: stem cells from human exfoliated deciduous teeth - PubMed (original) (raw)
SHED: stem cells from human exfoliated deciduous teeth
Masako Miura et al. Proc Natl Acad Sci U S A. 2003.
Abstract
To isolate high-quality human postnatal stem cells from accessible resources is an important goal for stem-cell research. In this study we found that exfoliated human deciduous tooth contains multipotent stem cells [stem cells from human exfoliated deciduous teeth (SHED)]. SHED were identified to be a population of highly proliferative, clonogenic cells capable of differentiating into a variety of cell types including neural cells, adipocytes, and odontoblasts. After in vivo transplantation, SHED were found to be able to induce bone formation, generate dentin, and survive in mouse brain along with expression of neural markers. Here we show that a naturally exfoliated human organ contains a population of stem cells that are completely different from previously identified stem cells. SHED are not only derived from a very accessible tissue resource but are also capable of providing enough cells for potential clinical application. Thus, exfoliated teeth may be an unexpected unique resource for stem-cell therapies including autologous stem-cell transplantation and tissue engineering.
Figures
Figure 1
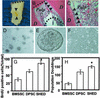
Isolation of SHED. (A) The exfoliated primary incisor contained dental pulp as shown (black triangles). The dashed line shows the occlusion edge of the incisor. (B and C) Hematoxylin/eosin staining indicated dentin (D) and pulp of exfoliated deciduous teeth. The pulp contained odontoblasts (arrows), blood vessels (open arrows), and connective tissues. The straight and curved dashed lines in B represent the occlusion and resorbed root surfaces, respectively. (D) Single colonies were formed after SHED were plated at low density and cultured for 2 weeks. (E) SHED were capable of forming sphere-like clusters when cultured with the conditions described in Materials and Methods. (F) The sphere-like clusters could be dissociated by passage through needles and subsequently grew on 0.1% gelatin-coated dishes. (G) The proliferation rates of SHED, BMSSCs, and DPSCs were assessed by BrdUrd (BrdU) incorporation for 12 h. SHED showed a significantly higher proliferation rate in comparison with BMSSCs and DPSCs (*, P < 0.05, Student's _t_ test). (_H_) SHED were able to proliferate to >140 population doublings, which was significantly higher (*, P < 0.05, Student's t test) than BMSSCs and DPSCs.
Figure 2
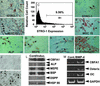
SHED possessed stem-cell characteristics. (A_–_E) The remnant pulp showed STRO-1 (open arrows in A) and CD146 (open arrows in B) immunopositive staining for cells in perivascular areas. Fluorescence-activated cell-sorting analysis showed that _ex vivo_-expanded SHED contained ≈9% STRO-1-positive cells (C). SHED expressed STRO-1 (D) and CD146 (E) (arrows). (F_–_I) SHED expressed the osteogenic and angiogenic markers ALP, MEPE, bFGF, and endostatin. (J and K) SHED were either cultured with regular medium (J) or
l
-ascorbate-2-phosphate, dexamethasone, and inorganic phosphate for 4 weeks (K). Alizarin red staining showed mineralized nodule formation in the induction (K). (L) Western blot analysis showed an up-regulated expression of CBFA1, ALP, MEPE, bone sialoprotein (BSP), and DSPP after the induction as described above. HSP90 was used to assess the amount of protein loaded per sample. (M) Human recombinant BMP-4 (300 ng/ml, 24 h) was added to induce a significant up-regulation of CBFA1, Osterix, and Osteocalcin (OC) in SHED as detected by semiquantitative PCR.
Figure 3
Transplanted SHED into immunocompromised mice. (A and B) After 8 weeks of transplantation, SHED were able to differentiate into odontoblasts (open arrows) that were responsible for the dentin-like structure (D) formation on the surfaces of HA (A). The same field is shown for human-specific alu in situ hybridization, indicating the human origin of odontoblasts (open arrows, B). The black dashed line represents interface between newly formed dentin (D) and HA/TCP (HA). (C) Immunohistochemical staining of anti-DSPP antibody shows a positive staining on the regenerated dentin (black arrows). (D) In contrast to DPSC transplants, newly generated bone (B) by host cells in the same SHED transplant shows no reactivity to the DSPP antibody. (E) Of 12 selected single-colony-derived SHED strains, only 3 (25%) were capable of generating dentin in vivo. Newly formed dentin (arrows) was found to be adjacent to the surfaces of HA/TCP carrier (HA) and associated with connective tissue (CT). (F) Human-specific alu in situ hybridization showed that human cells (open arrows) were associated with dentin formation (D) and were residing within the connective-tissue compartment (CT). (G) The remaining 75% (9 of 12) single-colony-derived SHED strains were unable to generate dentin in vivo. (H) In situ hybridization demonstrated that alu-positive human cells survived in the connective-tissue compartment (CT) in the transplants in which there was no odontogenesis. Human cells were also found to surround the blood vessels (arrows). (I) Seven of 12 (58.4%) single-colony-derived SHED lines induced a very limited amount of bone formation (B) on the surface of HA/TCP (HA). (J) Of 12 single-colony-derived SHED lines, 5 (41.6%) were able to induce a significant amount of bone formation (B) on the surfaces of HA/TCP (HA). (K) The alu in situ hybridization showed human cells (arrows) attached to the surfaces of HA/TCP (HA) at the initial site of bone formation (B). The black dashed lines represent the interface between newly formed bone (B) and HA/TCP (HA). (L) In situ hybridization studies showed the murine-specific pf1 DNA probe reacting with osteoblasts and osteocytes (arrows) associated with the new bone formation (B).
Figure 4
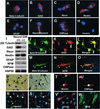
Neural differentiation of SHED. (A_–_H) Immunocytochemical staining depicts SHED expressing nestin, GFAP, NFM, CNPase, βIII-tubulin, GAD, and NeuN. (I) Western blot analysis confirmed that SHED expressed neural markers as described above. After 4 weeks of culture in the presence of B27 supplement, bFGF (40 ng/ml), and epidermal growth factor (20 ng/ml) (Neural Diff. +), expression levels of βIII-tubulin, GAD, and NeuN were up-regulated when compared with regular culture conditions as described in Materials and Methods (Neural Diff. −). However, expression levels of nestin, GFAP, CNPase, and NFM remained the same after the treatment. (J_–_O) SHED may coexpress neuronal markers including βIII-tubulin (green)/GAD (red) and βIII-tubulin (green)/NFM (red). The morphology of SHED showed elongated cell-cytoplasmic processes that sometimes coexpress neural markers (triangles) or only express individual neural marker (open arrows). (P_–_S) Toluidine blue (0.1%) staining depicting the altered morphology of SHED after induction with neural culture medium (P and Q, arrows). Immunopositive staining for anti-MAP2 and anti-Tau antibodies on dendrites and axon (R and S, arrows), respectively. Double-staining experiments showing βIII-tubulin-positive cells were also detected in the same field (R, triangle, green). (T_–_W) SHED continued to express glial cell markers including nestin (red), CNPase (red), GFAP (red), and NFM (green) by immunocytostaining.
Figure 5
Transplantation of SHED into the brain. (A) Diagram indicating injection of SHED into the dentate gyrus of the hippocampus. (B) SHED were cultured in the neural-differentiation medium as described in Materials and Methods for 1 week, after which 5,000 cells in 0.5 μl of PBS were injected into the dentate gyrus of the hippocampus of immunocompromised mice. After 10 days, the brain was fixed and prepared for immunofluorescence staining with NFM and human-specific anti-mitochondrial antibody. The anti-mitochondrial antibody immunostaining showed human SHED (arrows, green) in the dentate gyrus of the hippocampus with coexpression of NFM (arrows, red). In merged images, coexpression of human mitochondria and NFM showed colocalization of antigen expression as indicated by yellow fluorescence (arrows). (Magnification, ×20.)
Figure 6
Adipogenic differentiation of SHED. (A) Cultured SHED formed Oil red O-positive lipid clusters after 5 weeks of induction in the presence of 0.5 mM isobutylmethylxanthine, 0.5 μM hydrocortisone, and 60 μM indomethacin. (B) A significant up-regulation of peroxisome proliferator-activated receptor-γ2 (PPARγ2) and lipoprotein lipase (LPL) was observed in the group induced with the adipogenic mixture (Adip) as compared with the control group (Cont) by RT-PCR.
Similar articles
- [Isolation and identification of stem cells derived from human exfoliated deciduous teeth].
Xu N, Chen K, Shen YY. Xu N, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2009 Mar;29(3):479-82. Nan Fang Yi Ke Da Xue Xue Bao. 2009. PMID: 19304530 Chinese. - Stem Cells from Human Exfoliated Deciduous Teeth: A Growing Literature.
Martinez Saez D, Sasaki RT, Neves AD, da Silva MC. Martinez Saez D, et al. Cells Tissues Organs. 2016;202(5-6):269-280. doi: 10.1159/000447055. Epub 2016 Aug 20. Cells Tissues Organs. 2016. PMID: 27544531 Review. - Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells.
Nourbakhsh N, Soleimani M, Taghipour Z, Karbalaie K, Mousavi SB, Talebi A, Nadali F, Tanhaei S, Kiyani GA, Nematollahi M, Rabiei F, Mardani M, Bahramiyan H, Torabinejad M, Nasr-Esfahani MH, Baharvand H. Nourbakhsh N, et al. Int J Dev Biol. 2011;55(2):189-95. doi: 10.1387/ijdb.103090nn. Int J Dev Biol. 2011. PMID: 21671222 - Comparison of mesenchymal-like stem/progenitor cells derived from supernumerary teeth with stem cells from human exfoliated deciduous teeth.
Lee S, An S, Kang TH, Kim KH, Chang NH, Kang S, Kwak CK, Park HS. Lee S, et al. Regen Med. 2011 Nov;6(6):689-99. doi: 10.2217/rme.11.95. Regen Med. 2011. PMID: 22050521 - Dental stem cells: dentinogenic, osteogenic, and neurogenic differentiation and its clinical cell based therapies.
Brar GS, Toor RS. Brar GS, et al. Indian J Dent Res. 2012 May-Jun;23(3):393-7. doi: 10.4103/0970-9290.102239. Indian J Dent Res. 2012. PMID: 23059580 Review.
Cited by
- Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID-19 pandemics.
Yadav P, Vats R, Bano A, Bhardwaj R. Yadav P, et al. Life Sci. 2020 Dec 15;263:118588. doi: 10.1016/j.lfs.2020.118588. Epub 2020 Oct 10. Life Sci. 2020. PMID: 33049279 Free PMC article. Review. - Molecular differences between stromal cell populations from deciduous and permanent human teeth.
Kaukua N, Chen M, Guarnieri P, Dahl M, Lim ML, Yucel-Lindberg T, Sundström E, Adameyko I, Mao JJ, Fried K. Kaukua N, et al. Stem Cell Res Ther. 2015 Apr 18;6(1):59. doi: 10.1186/s13287-015-0056-7. Stem Cell Res Ther. 2015. PMID: 25927523 Free PMC article. - Roles of Dental Mesenchymal Stem Cells in the Management of Immature Necrotic Permanent Teeth.
Cui D, Yu S, Zhou X, Liu Y, Gan L, Pan Y, Zheng L, Wan M. Cui D, et al. Front Cell Dev Biol. 2021 May 19;9:666186. doi: 10.3389/fcell.2021.666186. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095133 Free PMC article. Review. - Therapeutic potential of stem cells from human exfoliated deciduous teeth infusion into patients with type 2 diabetes depends on basal lipid levels and islet function.
Li W, Jiao X, Song J, Sui B, Guo Z, Zhao Y, Li J, Shi S, Huang Q. Li W, et al. Stem Cells Transl Med. 2021 Jul;10(7):956-967. doi: 10.1002/sctm.20-0303. Epub 2021 Mar 4. Stem Cells Transl Med. 2021. PMID: 33660433 Free PMC article. - The regenerative medicine in oral and maxillofacial surgery: the most important innovations in the clinical application of mesenchymal stem cells.
Tatullo M, Marrelli M, Paduano F. Tatullo M, et al. Int J Med Sci. 2015 Jan 1;12(1):72-7. doi: 10.7150/ijms.10706. eCollection 2015. Int J Med Sci. 2015. PMID: 25552921 Free PMC article. Review.
References
- Spradling A, Drummond-Barbosa D, Kai T. Nature. 2001;414:98–104. - PubMed
- Gage F H. Science. 2000;287:1433–1438. - PubMed
- Prockop D J. Science. 1997;276:71–74. - PubMed
- Weissman I L. Science. 2000;287:1442–1446. - PubMed
- Pittenger M F, Mackay A M, Beck S C, Jaiswal R K, Douglas R, Mosca J D, Moorman M A, Simonetti D W, Craig S, Marshak D R. Science. 1999;284:143–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical