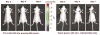Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice - PubMed (original) (raw)
Comparative Study
Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice
Abhijit De et al. Mol Ther. 2003 May.
Abstract
Lentiviral-mediated gene delivery holds significant promise for sustained gene expression within living systems. Vesicular stomatitis virus glycoprotein-pseudotyped human immunodeficiency virus type 1-based lentiviral vectors can be used to introduce transgenes in a broad spectrum of dividing as well as nondividing cells. In the current study, we construct a lentiviral vector carrying two reporter genes separated by an internal ribosomal entry site and utilize that virus in delivering both genes into neuroblastoma cells in cell culture and into cells implanted in living mice. We utilize two reporter genes, a mutant herpes simplex virus type 1 (HSV1) sr39tk as a reporter gene compatible with positron emission tomography (PET) and a bioluminescent optical reporter gene, firefly luciferase (Fluc), to image expression in living mice by an optical charge-coupled device (CCD) camera. By using this lentivirus, neuroblastoma (N2a) cells are stably transfected and a high correlation (R(2) = 0.91) between expressions of the two reporter genes in cell culture is established. Imaging of both reporter genes using microPET and optical CCD camera in living mice is feasible, with the optical approach being more sensitive, and a high correlation (R(2) = 0.86) between gene expressions is again observed in lentiviral-infected N2a tumor xenografts. Indirect imaging of HSV1-sr39tk suicide gene therapy utilizing Fluc is also feasible and can be detected with increased sensitivity by using the optical CCD. These preliminary results validate the use of lentiviral vectors carrying reporter genes for multimodality imaging of gene expression and should have many applications, including imaging of xenografts, metastasis, and cell trafficking as well as noninvasive monitoring of lentiviral-mediated gene delivery and expression.
Figures
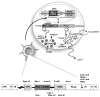
FIG. 1
Diagrammatic representation showing an IRES-based bicistronic lentivector used to link the activity of two reporter genes. The first gene (HSV1-sr39tk) is linked to the second gene (F_luc_) by an EMCV IRES sequence. When this combination of genes is packaged within a lentivector for cell targeting, the virus delivers this DNA cassette to the host cell nucleus followed by a reverse transcription reaction. The two genes are integrated within the host genome irrespective of the cell division state and start producing messenger RNA as the host genome replicates. These mRNA molecules are translated to proteins within the host cytoplasm. By using FHBG as a PET reporter probe one can quantitatively image the HSV1-sr39TK activity within living subjects. The same is also true for the F_luc_ gene, in that by administration of D-Luciferin, a substrate for FLUC, light production can be imaged.
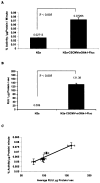
FIG. 2
HSV1-sr39TK and FLUC enzyme activity in lentiviral (CS-CMV_sr39tk_-IF_luc_)-infected N2a cells. The TK activity is expressed as percentage conversion of 8-3H-PCV to its phosphorylated form per microgram protein per minute. The FLUC activity is expressed as RLU per microgram protein per second. Error bars represent SEM for triplicate measurements. (A) Relative HSV1-sr39TK enzyme activity in lentiviral CS-CMV_sr39tk_-I-F_luc-_transduced N2a cells compared to parental N2a cells as a negative control. (B) Relative FLUC enzyme activity in lentiviral CS-CMV_sr39tk_-I-F_luc-_transduced N2a cells compared with parental N2a cells as a negative control. (C) Correlation between HSV1-sr39TK (y axis) and FLUC (x axis) enzyme activity in lentiviral CS-CMV_sr39tk_-I-F_luc-_transduced N2a cells as determined by assaying different numbers of infected cells. The correlation coefficient is _R_2 = 0.91.
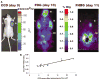
FIG. 3
Optical CCD and microPET imaging of a mouse implanted with N2a cells stably expressing HSV1-sr39tk and F_luc_ reporter genes. (A) Optical CCD image for F_luc_ expression on day 9, microPET FDG scan to check tumor viability on day 10, and microPET FHBG scan on day 11 for HSV1-sr39tk gene expression. Tumors were grown by injecting 5 × 106 N2a cells transduced in culture by lentivirus (CS-CMV_sr39tk_-I-F_luc_) (R) and control tumors an equal number of parental N2a cells (L). B, brain; GI, gastrointestinal tract; L, left tumor; R, right tumor. (B) Graph showing correlation of HSV1-sr39tk and F_luc_ gene expression in tumor xenografts. The correlation was established based on repeated scanning of four mice on days 8, 10, and 12 with both the microPET and the optical CCD camera. Individual mean FHBG %ID/g values versus maximum photons/s/cm2/sr values calculated from the regions of interest (ROI) on the respective scanned images of the tumor-bearing mice are plotted. Each of the 12 data points represents ROI values from bioluminescence (x axis) and microPET (y axis) images of the same mouse on the same day. The correlation coefficient is _R_2 = 0.86.
FIG. 4
Optical CCD images of tumor xenografts showing F_luc_ gene expression over time. Tumors were implanted sc with 5 × 105 lentiviral (CS-CMV_sr39tk_-I-F_luc_)-infected N2a cells on the right shoulder (R) and same number of parental N2a cells on the left shoulder (L) on day 0. The mouse was repeatedly scanned with the CCD on days 0, 4, 7, 11, and 14 as the tumors grew. The highest expression was observed on day 11, after which internal necrosis within the tumors likely resulted in lowered intensity of FLUC signal on day 14. All images shown are the visible light image superimposed on the optical CCD bioluminescence images.
FIG. 5
Monitoring of HSV1-sr39tk suicide gene therapy indirectly by F_luc_ reporter gene expression in a tumor xenograft model. In a set of six mice, control tumors (L) with N2a cells infected with lentivirus CS-CMVF_luc_ and experimental tumors (R) with N2a cells infected with lentivirus CS-CMV_sr39tk_-I-F_luc_ were implanted with 2 ×106 cells. Tumors were allowed to grow for 6 days before GCV treatment was started (day 0). GCV was administered daily at a dose of 25 mg/mouse/day. Follow-up scans with D-Luciferin on days 3 and 7 of GCV treatment showed significant decrease in the FLUC signal at the site of the experimental tumor (R), whereas the control tumor (L) continued to grow with increasing light signal.
FIG. 6
Optical CCD imaging of F_luc_ gene expression over 7 days in a living mouse carrying xenografts. On the left, tumors were implanted sc with parental N2a cells on both shoulders and allowed to grow to a palpable size of ~5 mm diameter before lentivirus (CS-CMV_sr39tk_-I-F_luc_) was injected directly into the right tumor (R) only once. The animals were scanned for F_luc_ expression on days 2, 4, and 7 after virus injection. On the right, tumors were implanted sc with parental N2a cells on the left shoulder (L) and with lentiviral (CS-CMV_sr39tk_-I-F_luc_)-infected N2a cells on the right shoulder (R). These animals were scanned on days 0 (2 h after cell implantation), 4, and 7.
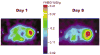
FIG. 7
FHBG microPET images of transverse sections of a mouse on days 1 and 8 with HSV1-sr39tk gene expression in a tumor xenograft. N2a parental cells were implanted sc on both shoulders and allowed to grow to a palpable size of ~5 mm diameter before lentivirus (CS-CMV_sr39tk_-I-F_luc_) was injected directly into the right tumor (R) only once. L indicates left tumor.
Similar articles
- Noninvasive monitoring of target gene expression by imaging reporter gene expression in living animals using improved bicistronic vectors.
Wang Y, Iyer M, Annala AJ, Chappell S, Mauro V, Gambhir SS. Wang Y, et al. J Nucl Med. 2005 Apr;46(4):667-74. J Nucl Med. 2005. PMID: 15809490 - Construction and validation of improved triple fusion reporter gene vectors for molecular imaging of living subjects.
Ray P, Tsien R, Gambhir SS. Ray P, et al. Cancer Res. 2007 Apr 1;67(7):3085-93. doi: 10.1158/0008-5472.CAN-06-2402. Cancer Res. 2007. PMID: 17409415 - Imaging tri-fusion multimodality reporter gene expression in living subjects.
Ray P, De A, Min JJ, Tsien RY, Gambhir SS. Ray P, et al. Cancer Res. 2004 Feb 15;64(4):1323-30. doi: 10.1158/0008-5472.can-03-1816. Cancer Res. 2004. PMID: 14973078 Free PMC article. - Herpes simplex virus thymidine kinase as a marker/reporter gene for PET imaging of gene therapy.
Blasberg RG, Tjuvajev JG. Blasberg RG, et al. Q J Nucl Med. 1999 Jun;43(2):163-9. Q J Nucl Med. 1999. PMID: 10429512 Review. - Monitoring gene therapy with reporter gene imaging.
Ray P, Bauer E, Iyer M, Barrio JR, Satyamurthy N, Phelps ME, Herschman HR, Gambhir SS. Ray P, et al. Semin Nucl Med. 2001 Oct;31(4):312-20. doi: 10.1053/snuc.2001.26209. Semin Nucl Med. 2001. PMID: 11710773 Review.
Cited by
- Regression of experimental NIS-expressing breast cancer brain metastases in response to radioiodide/gemcitabine dual therapy.
Renier C, Do J, Reyna-Neyra A, Foster D, De A, Vogel H, Jeffrey SS, Tse V, Carrasco N, Wapnir I. Renier C, et al. Oncotarget. 2016 Aug 23;7(34):54811-54824. doi: 10.18632/oncotarget.10238. Oncotarget. 2016. PMID: 27363025 Free PMC article. - Continuous infusion of UHMWPE particles induces increased bone macrophages and osteolysis.
Ren PG, Irani A, Huang Z, Ma T, Biswal S, Goodman SB. Ren PG, et al. Clin Orthop Relat Res. 2011 Jan;469(1):113-22. doi: 10.1007/s11999-010-1645-5. Clin Orthop Relat Res. 2011. PMID: 21042895 Free PMC article. - Noninvasive cell-tracking methods.
Kircher MF, Gambhir SS, Grimm J. Kircher MF, et al. Nat Rev Clin Oncol. 2011 Sep 27;8(11):677-88. doi: 10.1038/nrclinonc.2011.141. Nat Rev Clin Oncol. 2011. PMID: 21946842 Review. - A Novel Luciferase-Based Reporter Gene Technology for Simultaneous Optical and Radionuclide Imaging of Cells.
Gaspar N, Handula M, Stroet MCM, Marella-Panth K, Haeck J, Kirkland TA, Hall MP, Encell LP, Dalm S, Lowik C, Seimbille Y, Mezzanotte L. Gaspar N, et al. Int J Mol Sci. 2024 Jul 27;25(15):8206. doi: 10.3390/ijms25158206. Int J Mol Sci. 2024. PMID: 39125775 Free PMC article. - In vivo Noninvasive Small Animal Molecular Imaging.
Youn H, Hong KJ. Youn H, et al. Osong Public Health Res Perspect. 2012 Mar;3(1):48-59. doi: 10.1016/j.phrp.2012.02.002. Osong Public Health Res Perspect. 2012. PMID: 24159487 Free PMC article. Review.
References
- Verma IM, Somia N. Gene therapy—Promises, problems and prospects. Nature. 1997;389:239–242. - PubMed
- Yu D, et al. Prostate-specific targeting using PSA promoter-based lentiviral vectors. Cancer Gene Ther. 2001;8:628–635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R24 CA92865/CA/NCI NIH HHS/United States
- P50 CA86306/CA/NCI NIH HHS/United States
- P50 CA086306/CA/NCI NIH HHS/United States
- R0-1 CA82214/CA/NCI NIH HHS/United States
- R24 CA092865/CA/NCI NIH HHS/United States
- R01 CA082214/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical