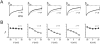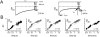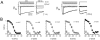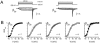Distinctive modulatory effects of five human auxiliary beta2 subunit splice variants on L-type calcium channel gating - PubMed (original) (raw)
Comparative Study
Distinctive modulatory effects of five human auxiliary beta2 subunit splice variants on L-type calcium channel gating
Shoji X Takahashi et al. Biophys J. 2003 May.
Abstract
Sequence analysis of the human genome permitted cloning of five Ca(2+)-channel beta(2) splice variants (beta(2a)-beta(2e)) that differed only in their proximal amino-termini. The functional consequences of such beta(2)-subunit diversity were explored in recombinant L-type channels reconstituted in HEK 293 cells. Beta(2a) and beta(2e) targeted autonomously to the plasma membrane, whereas beta(2b)-beta(2d) localized to the cytosol when expressed in HEK 293 cells. The pattern of modulation of L-type channel voltage-dependent inactivation gating correlated with the subcellular localization of the component beta(2) variant-membrane-bound beta(2a) and beta(2e) subunits conferred slow(er) channel inactivation kinetics and displayed a smaller fraction of channels recovering from inactivation with fast kinetics, compared to beta(2b)-beta(2d) channels. The varying effects of beta(2) subunits on inactivation gating were accounted for by a quantitative model in which L-type channels reversibly distributed between fast and slow forms of voltage-dependent inactivation-membrane-bound beta(2) subunits substantially decreased the steady-state fraction of fast inactivating channels. Finally, the beta(2) variants also had distinctive effects on L-type channel steady-state activation gating, as revealed by differences in the waveforms of tail-activation (G-V) curves, and conferred differing degrees of prepulse facilitation to the channel. Our results predict important physiological consequences arising from subtle changes in Ca(2+)-channel beta(2)-subunit structure due to alternative splicing and emphasize the utility of splice variants in probing structure-function mechanisms.
Figures
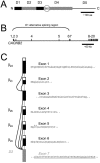
FIGURE 1
Genomic perspective clarifies basis of Ca2+ channel _β_2-subunit molecular diversity. (A) Modular domain structure proposed for Ca2+-channel β subunits based on primary sequence identity. Two conserved domains (D2 and D4, gray boxes) are interspersed by three variable domains (D1, D3, and D5). D1 is an important determinant of channel inactivation, and D4 contains the _β_-interaction domain, important for interactions with pore-forming _α_1-subunits. Alternative splicing of _β_2 is known to occur in at least two regions, D1 and D3 (black boxes). (B) Schematic structure of human CACNB2 based on analysis of human genomic DNA sequence. Exons are represented by vertical lines and introns by horizontal lines; introns are approximately to scale, whereas exons are not to scale. (C) Schematic of mutually exclusive splicing patterns of exons 1–6 that result in the generation of five alternative _β_2-subunit D1 domains. The corresponding amino acid sequences are represented. Exons 1, 2, and 4–6 encode the amino-termini of the distinct _β_2 splice variants; each has their own initiation codon and 5′ untranslated region (white box). Exon 3 is common to _β_2c and _β_2d. Exon 7 (gray box, with underlined sequence) is a constitutive exon that encodes the beginning of the D2 domain common to all _β_2 variants.
FIGURE 2
Recombinant human _β_2 splice variants target differentially in HEK 293 cells. (A) Confocal, and (B) line-scan images of _β_2-GFP fusion proteins. _β_2a and _β_2e splice variants were localized to the plasma membrane; membrane targeting of _β_2a is caused by palmitoylation of two cysteines in D1. In contrast, _β_2b–_β_2d variants were distributed throughout the cytosol but excluded from the nucleus. F is the normalized fluorescence intensity through the midpoint of images in A.
FIGURE 3
_β_2 splice variants confer distinctive inactivation kinetics to recombinant L-type channels. (A) Normalized and averaged Ba2+ currents evoked by 1-s depolarizations to 0 mV. All currents had biexponential inactivation kinetics; those reconstituted with _β_2a and _β_2e had markedly slow inactivation kinetics compared to _β_2b–_β_2d. (B) Voltage-dependence of fast inactivation. The fraction of current remaining 300 ms into the test pulse, _r_300, was computed as an index of inactivation. Higher _r_300 values reflect a slower rate of inactivation. Inactivation conferred by _β_2a and _β_2e was markedly less voltage-dependent compared to _β_2b–_β_2d. In both A and B, _β_2a data are reproduced as gray lines in _β_2b–_β_2e panels to permit direct visual comparison.
FIGURE 4
L-type channel recovery from inactivation occurs with distinct fast and slow components, but with differing amplitudes, across _β_2 splice variants. (A) Exemplar current traces from _β_2a and _β_2b channels evoked by a traditional recovery from inactivation protocol in which a 1-s prepulse to 0 mV is followed by a 50-ms test pulse to 0 mV at variable interpulse durations. Here and throughout, _β_2a and _β_2b exemplars are shown as representative slow- and fast-inactivating channels, respectively. (B) Plots of the fractional recovery from inactivation, RF. Data points were calculated as RF = (_I_test − _I_end)/(_I_prepulse − _I_end). Peak prepulse currents decreased by ∼20% for all _β_2 variants by the end of the recovery protocol. This rundown was systematically accounted for in the analysis by normalizing all currents to the peak prepulse current from the first sweep. Smooth black curves through the data are biexponential fits generated from the equation presented in Methods. Curves were constrained to have the same fast- and slow- time constants, while the amplitudes of fast and slow components were determined by least-squares criteria (parameter values are presented in Table 1). Biexponential fits to _β_2a data are reproduced in the other panels (gray curves) to facilitate visual comparison of the kinetics of recovery from inactivation among the _β_2 splice variants. _β_2a and _β_2e had a smaller fraction of channels recovering from inactivation with fast kinetics compared to _β_2b–_β_2d channels.
FIGURE 5
L-type channel steady-state inactivation properties are relatively insensitive to the identity of the component _β_2-subunit splice variant. (A) Exemplar current traces from _β_2a and _β_2b channels evoked by a traditional steady-state inactivation protocol in which a 10-ms normalizing pulse to 0 mV is followed sequentially by a variable-voltage family of 20-s conditioning pulses and a 100-ms test pulse to 0 mV. Only the first 200 ms of the currents during the conditioning depolarizations is shown. Traces shown were obtained with conditioning pulse voltages of 0, −30, −50, and −80 mV. (B) Plots of steady-state inactivation, h(20 s), as a function of voltage of the conditioning pulse. The normalized data points were obtained from the peak values of the prepulse and test currents, repectively, as h(20 s) = _I_test/_I_prepulse. Smooth black curve through the _β_2a data was generated from a single Boltzmann function as described in Methods, with parameter values given in Table 1. Reproduction of the _β_2a Boltzmann fit onto the panels for other _β_2 splice variants (gray curves) demonstrated marked agreement with these other data, indicating little difference among the distinct _β_2 variants.
FIGURE 6
Different _β_2 splice variants impart distinctive steady-state activation behavior to L-type channels. (A) Exemplar current traces from _β_2a and _β_2b channels evoked by a traditional tail-activation protocol in which channels were steady-state activated by a variable-voltage family of 20-ms test pulses, and tail currents measured after repolarization to −50 mV. Traces shown were obtained with test-pulse voltages of −20, 0, +40, and +100 mV. (B) Normalized (G/_G_max) G-V curves for channels incorporating the different _β_2 variants. Smooth black curves through the data were generated from dual-Boltzmann functions as described in Methods, with parameter values given in Table 1. To generate fits, _V_1/2 and k values were constrained to the values given in the legend to Table 1, and the amplitudes of the low- and high-threshold components determined by least-squares criteria. The reproduced fits from _β_2a data (gray curves) displayed varying degrees of difference from the data for other _β_2 variants, visually affirming that distinct _β_2 variants gave rise to differing fractions of channels that operated in the low threshold of activation regime.
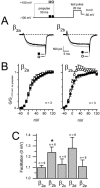
FIGURE 7
_β_2 splice variants differentially affect prepulse facilitation of recombinant L-type channels. (A) Alternating prepulse voltage protocol (above) and exemplar Ba2+ test-pulse currents (below) recorded from L-type channels reconstituted with _β_2a (left) or _β_2b (right). Currents were evoked by a 20-ms test-pulse to 0 mV in the absence of prepulse (black traces) or after a 50-ms prepulse to +100 mV (gray traces); the interpulse duration was 50 ms. Tail currents were analyzed at −50 mV repolarization potential. (B) Normalized and averaged G-V curves generated from alternating-prepulse protocol for L-type channels reconstituted with _β_2a (left, ▪, □) and _β_2b (right, •, ○). Tail-current amplitudes were normalized to the current evoked at +110 mV in the absence of prepulse and plotted as a function of test-pulse potential, which was varied from −40 to +120 mV in 10-mV increments. Solid symbols (▪, •) denote currents evoked in the absence of prepulse; open symbols (□, ○) denote currents evoked after a prepulse. (C) Comparison of facilitation measured at a test-pulse potential of 0 mV across all five _β_2 splice variants. Facilitation was calculated as the tail-current amplitude after a prepulse, normalized to the tail-current amplitude in the absence of prepulse. The difference between _β_2a and _β_2b was statistically significant (asterisk denotes P < 0.05, Student's _t_-test).
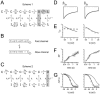
FIGURE 8
Quantitative descriptions of L-type channel inactivation gating profiles as conferred by distinct _β_2 splice variants. (A_–_C) Alternative kinetic schemes that could account for the existence of two kinetically distinct inactivation states, which are suggested experimentally by the biexponential kinetics of channel recovery from inactivation. In Fig. 8 A, Scheme 1, inactive states were separated into those connected to the activation pathway with either fast or slow entry/exit rates (_I_f and _I_s, respectively). In Fig. 8 C, Scheme 2, inactivation proceeds from the open state by only one pathway. In this case, the biexponential kinetics of recovery from inactivation arise from the existence of two channel populations (or modes) displaying kinetically distinct fast and slow modes of inactivation. (D_–_F) Simulations of inactivation gating of _β_2a and _β_2b channels using Fig. 8 C, Scheme 2 showed marked agreement between the model (black curves) and experimental data (gray curves, and ▪, ○). Values of rate constants used in simulations are presented in Table 2. In simulations, the fraction of fast channels was given by the corresponding _F_fast values, as determined from recovery from inactivation protocols (Table 2). Note that for simulations of _β_2a and _β_2b channels, rate constants were identical in the two cases. Hence, all the differences between the channel types were completely accounted for by the differing values of the fraction of fast inactivating channels, _F_fast. (G) In the case of steady-state inactivation, simulations using the dual-population model as described above (dotted lines) diverged from the experimental data (▪, ○). Remarkably, when it was assumed that all the channels were gating in the slow mode, model predictions of steady-state inactivation (black curves) superimposed the experimental data. This suggested that during the ultra-long 20-s conditioning pulses used in steady-state inactivation protocols, channels consolidate into the slow inactivating mode. Evidence of such consolidation has been experimentally observed.
Similar articles
- Voltage-gated rearrangements associated with differential beta-subunit modulation of the L-type Ca(2+) channel inactivation.
Kobrinsky E, Kepplinger KJ, Yu A, Harry JB, Kahr H, Romanin C, Abernethy DR, Soldatov NM. Kobrinsky E, et al. Biophys J. 2004 Aug;87(2):844-57. doi: 10.1529/biophysj.104.041152. Biophys J. 2004. PMID: 15298893 Free PMC article. - A novel molecular inactivation determinant of voltage-gated CaV1.2 L-type Ca2+ channel.
Livneh A, Cohen R, Atlas D. Livneh A, et al. Neuroscience. 2006;139(4):1275-87. doi: 10.1016/j.neuroscience.2006.01.028. Epub 2006 Mar 14. Neuroscience. 2006. PMID: 16533566 - Gene splicing of an invertebrate beta subunit (LCavβ) in the N-terminal and HOOK domains and its regulation of LCav1 and LCav2 calcium channels.
Dawson TF, Boone AN, Senatore A, Piticaru J, Thiyagalingam S, Jackson D, Davison A, Spafford JD. Dawson TF, et al. PLoS One. 2014 Apr 1;9(4):e92941. doi: 10.1371/journal.pone.0092941. eCollection 2014. PLoS One. 2014. PMID: 24690951 Free PMC article. - Voltage-dependent calcium channels.
Lacinová L. Lacinová L. Gen Physiol Biophys. 2005 Jun;24 Suppl 1:1-78. Gen Physiol Biophys. 2005. PMID: 16096350 Review. - Structure and function of the β subunit of voltage-gated Ca²⁺ channels.
Buraei Z, Yang J. Buraei Z, et al. Biochim Biophys Acta. 2013 Jul;1828(7):1530-40. doi: 10.1016/j.bbamem.2012.08.028. Epub 2012 Sep 7. Biochim Biophys Acta. 2013. PMID: 22981275 Free PMC article. Review.
Cited by
- A membrane-associated phosphoswitch in Rad controls adrenergic regulation of cardiac calcium channels.
Papa A, Del Rivero Morfin PJ, Chen BX, Yang L, Katchman AN, Zakharov SI, Liu G, Bohnen MS, Zheng V, Katz M, Subramaniam S, Hirsch JA, Weiss S, Dascal N, Karlin A, Pitt GS, Colecraft HM, Ben-Johny M, Marx SO. Papa A, et al. J Clin Invest. 2024 Jan 16;134(5):e176943. doi: 10.1172/JCI176943. J Clin Invest. 2024. PMID: 38227371 Free PMC article. - LITAF (Lipopolysaccharide-Induced Tumor Necrosis Factor) Regulates Cardiac L-Type Calcium Channels by Modulating NEDD (Neural Precursor Cell Expressed Developmentally Downregulated Protein) 4-1 Ubiquitin Ligase.
Moshal KS, Roder K, Kabakov AY, Werdich AA, Chiang DY, Turan NN, Xie A, Kim TY, Cooper LL, Lu Y, Zhong M, Li W, Terentyev D, Choi BR, Karma A, MacRae CA, Koren G. Moshal KS, et al. Circ Genom Precis Med. 2019 Sep;12(9):407-420. doi: 10.1161/CIRCGEN.119.002641. Epub 2019 Aug 28. Circ Genom Precis Med. 2019. PMID: 31462068 Free PMC article. - Atomic Mechanisms of Timothy Syndrome-Associated Mutations in Calcium Channel Cav1.2.
Korkosh VS, Kiselev AM, Mikhaylov EN, Kostareva AA, Zhorov BS. Korkosh VS, et al. Front Physiol. 2019 Mar 29;10:335. doi: 10.3389/fphys.2019.00335. eCollection 2019. Front Physiol. 2019. PMID: 30984024 Free PMC article. - Regulation of voltage-dependent calcium channels by RGK proteins.
Yang T, Colecraft HM. Yang T, et al. Biochim Biophys Acta. 2013 Jul;1828(7):1644-54. doi: 10.1016/j.bbamem.2012.10.005. Epub 2012 Oct 10. Biochim Biophys Acta. 2013. PMID: 23063948 Free PMC article. Review. - Orientation of palmitoylated CaVbeta2a relative to CaV2.2 is critical for slow pathway modulation of N-type Ca2+ current by tachykinin receptor activation.
Mitra-Ganguli T, Vitko I, Perez-Reyes E, Rittenhouse AR. Mitra-Ganguli T, et al. J Gen Physiol. 2009 Nov;134(5):385-96. doi: 10.1085/jgp.200910204. J Gen Physiol. 2009. PMID: 19858358 Free PMC article.
References
- Ball, S. L., P. A. Powers, H. S. Shin, C. W. Morgans, N. S. Peachey, and R. G. Gregg. 2002. Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest. Ophthalmol. Vis. Sci. 43:1595–1603. - PubMed
- Berjukow, S., R. Marksteiner, S. Sokolov, R. G. Weiss, E. Margreiter, and S. Hering. 2001. Amino acids in segment IVS6 and β-subunit interaction support distinct conformational changes during Ca(v)2.1 inactivation. J. Biol. Chem. 276:17076–17082. - PubMed
- Birnbaumer, L., N. Qin, R. Olcese, E. Tareilus, D. Platano, J. Costantin, and E. Stefani. 1998. Structures and functions of calcium channel β subunits. J. Bioenerg. Biomembr. 30:357–375. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous