NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease - PubMed (original) (raw)
NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease
Du-Chu Wu et al. Proc Natl Acad Sci U S A. 2003.
Abstract
Parkinson's disease (PD) is a neurodegenerative disorder of uncertain pathogenesis characterized by a loss of substantia nigra pars compacta (SNpc) dopaminergic (DA) neurons, and can be modeled by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Both inflammatory processes and oxidative stress may contribute to MPTP- and PD-related neurodegeneration. However, whether inflammation may cause oxidative damage in MPTP and PD is unknown. Here we show that NADPH-oxidase, the main reactive oxygen species (ROS)-producing enzyme during inflammation, is up-regulated in SNpc of human PD and MPTP mice. These changes coincide with the local production of ROS, microglial activation, and DA neuronal loss seen after MPTP injections. Mutant mice defective in NADPH-oxidase exhibit less SNpc DA neuronal loss and protein oxidation than their WT littermates after MPTP injections. We show that extracellular ROS are a main determinant in inflammation-mediated DA neurotoxicity in the MPTP model of PD. This study supports a critical role for NADPH-oxidase in the pathogenesis of PD and suggests that targeting this enzyme or enhancing extracellular antioxidants may provide novel therapies for PD.
Figures
Figure 1
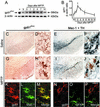
(A_–_D) RT-PCR shows ventral midbrain gp91phox, p67phox, and Mac-1 mRNA levels in saline-injected (S) and MPTP-injected mice from 0 to 14 days after injections. SNpc gp91phox mRNA labeling is negligible in saline-injected mice (E), whereas it is copious in MPTP-injected mice at 2 days (F). *, P < 0.05; **, P < 0.001, more than saline-treated mice (n = 4–6 per time point). (Scale, 2.5 mm.)
Figure 2
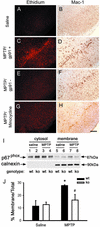
Western blot shows the time-dependent induction of gp91phox in mouse ventral midbrain after MPTP injections. +, Mouse macrophage lysate; s, saline. In saline-injected mice, gp91phox immunoreactivity (C and D, brown) is mild and localized in resting microglia, which are not abundant in the SNpc, as shown (E and F) by Mac-1 labeling (brown, arrow) and are intermingled with TH-positive neurons (gray-blue). Two days after MPTP injections, numerous gp91phox-positive cells are seen in the SNpc (G and H). These cells resemble activated microglial cells (H vs. J, arrow). At this point there are many fewer TH-positive neurons (I and J, arrowhead). Confocal microscopy shows that all gp91phox-positive cells are Mac-1-positive, thus confirming their microglial origin (K and L). Conversely, no gp91phox-positive cells are glial fibrillary acidic protein-positive cells, thus excluding their astrocyctic origin (N–P). *, P < 0.05, more than saline-treated mice (n = 6 per time point). [Scale bar, 2.5 mm (C, E, G, and I); 0.25 mm (D, F, H, and J); and 0.2 mm (K_–_P).]
Figure 3
(A) Representative Western blots illustrating the increase in ventral midbrain gp91phox protein content in two PD and two controls. (B) Bar graph showing mean Western blot gp91phox/β-actin ratios ± SEM for six PD and three control ventral midbrain samples. (C and D) Representative gp91phox immunostaining that shows positive cells in PD samples (arrowhead, gray-blue, membrane labeling) colocalizing with the microglial marker CD68 (arrow, red, cytosol labeling), but not with neuromelanin (brown pigment). *, P < 0.05, higher than controls. (Scale bar, 0.5 mm.)
Figure 4
Ethidium fluorescence (A) and Mac-1 immunostaining (B) are minimal in the saline-treated mice. By 2 days after MPTP injections, SNpc ethidium fluorescence is increased in WT mice (C) and is absent in gp91phox-deficient mice (E) and minocycline-treated WT mice (G). Microglial activation is prevented by minocycline (H) but is normal in gp91phox-deficient mice (E). MPTP stimulates NADPH-oxidase activation, as evidenced by p67phox translocation from the cytosol to the plasma membrane in WT mice (wt), but not in gp91phox-deficient mice (ko) (I and J); the membrane protein calnexin is used to normalize the data. Data are means ± SEM for four to six samples per group. *, P < 0.05, higher than controls; #, P < 0.05, less than MPTP-injected WT mice, but not different from both saline-injected groups.
Figure 5
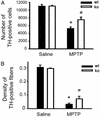
Stereological counts of TH-positive neurons in the SNpc (A) and optical density of striatal DA fibers (B) are higher in gp91phox-deficient mice (ko) compared with their WT littermates (wt) 7 days after MPTP injections (n = 4–8 samples per group). *, P < 0.05, less than saline-injected mice; #, P < 0.05, higher than MPTP-injected WT mice.
Figure 6
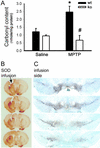
(A) Ventral midbrain carbonyl content, used as a marker of protein oxidative damage, is increased at 2 days after MPTP injections in WT mice (wt), but not in gp91phox-deficient mice (ko). Infusion of SOD1 into the left striatum attenuates the striatal (B) and the SNpc lesion on the infused side, but not on the contralateral, noninfused side (C) after a systemic injection of MPTP. *, P < 0.05, higher than controls; #, P < 0.05, less than MPTP-injected WT mice, but not different from the two saline-injected groups.
Similar articles
- Squamosamide derivative FLZ protects dopaminergic neurons against inflammation-mediated neurodegeneration through the inhibition of NADPH oxidase activity.
Zhang D, Hu X, Wei SJ, Liu J, Gao H, Qian L, Wilson B, Liu G, Hong JS. Zhang D, et al. J Neuroinflammation. 2008 May 28;5:21. doi: 10.1186/1742-2094-5-21. J Neuroinflammation. 2008. PMID: 18507839 Free PMC article. - Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase.
Zhang W, Wang T, Qin L, Gao HM, Wilson B, Ali SF, Zhang W, Hong JS, Liu B. Zhang W, et al. FASEB J. 2004 Mar;18(3):589-91. doi: 10.1096/fj.03-0983fje. Epub 2004 Jan 20. FASEB J. 2004. PMID: 14734632 - Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease.
Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Wu DC, et al. J Neurosci. 2002 Mar 1;22(5):1763-71. doi: 10.1523/JNEUROSCI.22-05-01763.2002. J Neurosci. 2002. PMID: 11880505 Free PMC article. - Nitric oxide and reactive oxygen species in Parkinson's disease.
Tieu K, Ischiropoulos H, Przedborski S. Tieu K, et al. IUBMB Life. 2003 Jun;55(6):329-35. doi: 10.1080/1521654032000114320. IUBMB Life. 2003. PMID: 12938735 Review. - Oxidative stress and regulated cell death in Parkinson's disease.
Dionísio PA, Amaral JD, Rodrigues CMP. Dionísio PA, et al. Ageing Res Rev. 2021 May;67:101263. doi: 10.1016/j.arr.2021.101263. Epub 2021 Feb 1. Ageing Res Rev. 2021. PMID: 33540042 Review.
Cited by
- Minocycline Rescues from Zinc-Induced Nigrostriatal Dopaminergic Neurodegeneration: Biochemical and Molecular Interventions.
Kumar V, Singh BK, Chauhan AK, Singh D, Patel DK, Singh C. Kumar V, et al. Mol Neurobiol. 2016 Jul;53(5):2761-2777. doi: 10.1007/s12035-015-9137-y. Epub 2015 Mar 13. Mol Neurobiol. 2016. PMID: 25764516 - Aging, Angiotensin system and dopaminergic degeneration in the substantia nigra.
Labandeira-Garcia JL, Rodriguez-Pallares J, Villar-Cheda B, Rodríguez-Perez AI, Garrido-Gil P, Guerra MJ. Labandeira-Garcia JL, et al. Aging Dis. 2011 Jun;2(3):257-74. Epub 2011 Apr 20. Aging Dis. 2011. PMID: 22396877 Free PMC article. - Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders.
Spaas J, van Veggel L, Schepers M, Tiane A, van Horssen J, Wilson DM 3rd, Moya PR, Piccart E, Hellings N, Eijnde BO, Derave W, Schreiber R, Vanmierlo T. Spaas J, et al. Cell Mol Life Sci. 2021 May;78(10):4615-4637. doi: 10.1007/s00018-021-03802-0. Epub 2021 Mar 10. Cell Mol Life Sci. 2021. PMID: 33751149 Free PMC article. Review. - Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection.
Cheng CY, Barro L, Tsai ST, Feng TW, Wu XY, Chao CW, Yu RS, Chin TY, Hsieh MF. Cheng CY, et al. Int J Mol Sci. 2021 Mar 16;22(6):3037. doi: 10.3390/ijms22063037. Int J Mol Sci. 2021. PMID: 33809762 Free PMC article. - Tauroursodeoxycholic Acid Protects Against Mitochondrial Dysfunction and Cell Death via Mitophagy in Human Neuroblastoma Cells.
Fonseca I, Gordino G, Moreira S, Nunes MJ, Azevedo C, Gama MJ, Rodrigues E, Rodrigues CMP, Castro-Caldas M. Fonseca I, et al. Mol Neurobiol. 2017 Oct;54(8):6107-6119. doi: 10.1007/s12035-016-0145-3. Epub 2016 Oct 3. Mol Neurobiol. 2017. PMID: 27699602
References
- Fahn S, Przedborski S. In: Merritt's Neurology. Rowland L P, editor. New York: Lippincott; 2000. pp. 679–693.
- Przedborski S, Kostic V, Giladi N, Eidelberg D. In: Dopamine Receptors and Transporters. Sidhu A, Laruelle M, Vernier P, editors. New York: Dekker; 2003. pp. 363–402.
- Chen H, Zhang S M, Hernan M A, Schwarzschild M A, Willett W C, Colditz G A. Movement Disorders. 2002;17,(Suppl. 5):S143.
- Gao H M, Jiang J, Wilson B, Zhang W, Hong J S, Liu B. J Neurochem. 2002;81:1285–1297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG13966/AG/NIA NIH HHS/United States
- NS37345/NS/NINDS NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- R01 AG013966/AG/NIA NIH HHS/United States
- NS38370/NS/NINDS NIH HHS/United States
- NS11766-27AI/NS/NINDS NIH HHS/United States
- P01 NS011766/NS/NINDS NIH HHS/United States
- NS38586/NS/NINDS NIH HHS/United States
- R01 NS042269/NS/NINDS NIH HHS/United States
- R01 NS038586/NS/NINDS NIH HHS/United States
- NS42269/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous